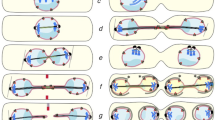
Abstract
Saccharomyces cerevisiae has been widely used as a model system for the study of basic biological processes which are usually evolutionarily conserved from yeasts to multicellular eukaryotes. These studies are very important because they shed light on mechanisms that are altered in human diseases and help the development of new biomarkers and therapies. The mitotic spindle is a conserved apparatus that governs chromosome segregation during mitosis. Given its crucial role for genome stability and, therefore, for cell viability, its structure and function are strictly regulated. Recent findings reveal new levels of regulation in mitotic spindle dynamics and link spindle pole diversification with cell fate determination, health, disease and aging.

Similar content being viewed by others
References
Asano S, Park JE, Sakchaisri K, Yu LR, Song S, Supavilai P, Veenstra TD, Lee KS (2005) Concerted mechanism of Swe1/Wee1 regulation by multiple kinases in budding yeast. EMBO J 24(12):2194–2204
Botchkarev VV Jr, Haber JE (2018) Functions and regulation of the Polo-like kinase Cdc5 in the absence and presence of DNA damage. Curr Genet 64(1):87–96
Carvalho P, Gupta ML Jr, Hoyt MA, Pellman D (2004) Cell cycle control of kinesin-mediated transport of Bik1 (CLIP-170) regulates microtubule stability and dynein activation. Dev Cell 6(6):815–829
Carvalho P, Tirnauer JS, Pellman D (2003) Surfing on microtubule ends. Trends Cell Biol 13(5):229–237
Caydasi AK, Pereira G (2012) SPOC alert when chromosomes get the wrong direction. Exp Cell Res 318(12):1421–1427
Chen XP, Yin H, Huffaker TC (1998) The yeast spindle pole body component Spc72p interacts with Stu2p and is required for proper microtubule assembly. J Cell Biol 141:1169–1179
Dhatchinamoorthy K, Mattingly M, Gerton JL (2018) Regulation of kinetochore configuration during mitosis. Curr Genet 64(6):1197–1203
Fraschini R (2017) Factors that control mitotic spindle dynamics. Adv Exp Med Biol 925:89–101
Fraschini R (2019) Divide precisely and proliferate safely: lessons from budding yeast. Front Genet 9:738
Fraschini R, Venturetti M, Chiroli E, Piatti S (2008) The spindle position checkpoint: how to deal with spindle misalignment during asymmetric cell division in budding yeast. Biochem Soc Trans 36(Pt 3):416–420
Hartwell LH, Mortimer RK, Culotti J, Culotti M (1973) Genetic control of the cell division cycle in yeast: V. Genetic analysis of cdc mutants. Genetics 74(2):267–286
Harvey SL, Charlet A, Haas W, Gygi SP, Kellogg DR (2005) Cdk1-dependent regulation of the mitotic inhibitor Wee1. Cell 122(3):407–420
Heil-Chapdelaine RA, Oberle JR, Cooper JA (2000) The cortical protein Num1p is essential for dynein-dependent interactions of microtubules with the cortex. J Cell Biol 151(6):1337–1344
Hotz M, Lengefeld J, Barral Y (2012) The MEN mediates the effects of the spindle assembly checkpoint on Kar9-dependent spindle pole body inheritance in budding yeast. Cell Cycle 11(16):3109–3116
Januschke J, Llamazares S, Reina J, Gonzalez C (2011) Drosophila neuroblasts retain the daughter centrosome. Nat Commun 2:243
Katsumata K, Nishi E, Afrin S, Narusawa K, Yamamoto A (2017) Position matters: multiple functions of LINC-dependent chromosome positioning during meiosis. Curr Genet 63(6):1037–1052
Larti F, Kahrizi K, Musante L, Hu H, Papari E, Fattahi Z, Bazazzadegan N, Liu Z, Banan M, Garshasbi M, Wienker TF, Ropers HH, Galjart N, Najmabadi H (2015) A defect in the CLIP1 gene (CLIP-170) can cause autosomal recessive intellectual disability. Eur J Hum Genet 23(3):331–336
Lee L, Tirnauer JS, Li J, Schuyler SC, Liu JY, Pellman D (2000) Positioning of the mitotic spindle by a cortical-microtubule capture mechanism. Science 287(5461):2260–2262
Lengefeld J, Hotz M, Rollins M, Baetz K, Barral Y (2017) Budding yeast Wee1 distinguishes spindle pole bodies to guide their pattern of age-dependent segregation. Nat Cell Biol 19(8):941–951
Lengefeld J, Yen E, Chen X, Leary A, Vogel J, Barral Y (2018) Spatial cues and not spindle pole maturation drive the asymmetry of astral microtubules between new and preexisting spindle poles. Mol Biol Cell 29(1):10–28
Lerit DA, Rusan NM (2013) PLP inhibits the activity of interphase centrosomes to ensure their proper segregation in stem cells. J Cell Biol 202(7):1013–1022
Lew DJ (2003) The morphogenesis checkpoint: how yeast cells watch their figures. Curr Opin Cell Biol 15:648–653
Li W, Saud SM, Young MR, Chen G, Hua B (2015) Targeting AMPK for cancer prevention and treatment. Oncotarget 6(10):7365–7378
Liakopoulos D, Kusch J, Grava S, Vogel J, Barral Y (2003) Asymmetric loading of Kar9 onto spindle poles and microtubules ensures proper spindle alignment. Cell 112(4):561–574
Lin H, de Carvalho P, Kho D, Tai CY, Pierre P, Fink GR, Pellman D (2001) Polyploids require Bik1 for kinetochore-microtubule attachment. J Cell Biol 155(7):1173–1184
Makrantoni V, Corbishley SJ, Rachidi N, Morrice NA, Robinson DA, Stark MJ (2014) Phosphorylation of Sli15 by Ipl1 is important for proper CPC localization and chromosome stability in Saccharomyces cerevisiae. PLoS One 9(2):e89399
Moore JK, D’Silva S, Miller RK (2006) The CLIP-170 homologue Bik1p promotes the phosphorylation and asymmetric localization of Kar9p. Mol Biol Cell 17(1):178–191
Nakajima Y, Cormier A, Tyers RG, Pigula A, Peng Y, Drubin DG, Barnes G (2011) Ipl1/aurora-dependent phosphorylation of Sli15/INCENP regulates CPC-spindle interaction to ensure proper microtubule dynamics. J Cell Biol 194(1):137–153
Pal G, Paraz MT, Kellogg DR (2008) Regulation of Mih1/Cdc25 by protein phosphatase 2A and casein kinase 1. J Cell Biol 180:931–945
Palou R, Palou G, Quintana DG (2017) A role for the spindle assembly checkpoint in the DNA damage response. Curr Genet 63(2):275–280
Pearson CG, Bloom K (2004) Dynamic microtubules lead the way for spindle positioning. Nat Rev Mol Cell Biol 5(6):481–492
Raspelli E, Cassani C, Chiroli E, Fraschini R (2015) Budding yeast Swe1 is involved in the control of mitotic spindle elongation and is regulated by Cdc14 phosphatase during mitosis. J Biol Chem 290(1):1–12
Raspelli E, Facchinetti S, Fraschini R (2018) Novel insights into Swe1 and Mih1 role in the regulation of mitotic spindle dynamics. J Cell Sci 131(17):1–13
Russell P, Moreno S, Reed SI (1989) Conservation of mitotic controls in fission and budding yeasts. Cell 57(2):295–303
Siller KH, Doe CQ (2009) Spindle orientation during asymmetric cell division. Nat Cell Biol 11(4):365–374
Stangier MM, Kumar A, Chen X, Farcas AM, Barral Y, Steinmetz MO (2018) Structure–function relationship of the Bik1–Bim1 complex. Structure 26(4):607–618
Tame MA, Raaijmakers JA, Afanasyev P, Medema RH (2016) Chromosome misalignments induce spindle-positioning defects. EMBO Rep 17(3):317–325
Tripodi F, Fraschini R, Zocchi M, Reghellin V, Coccetti P (2018) Snf1/AMPK is involved in the mitotic spindle alignment in Saccharomyces cerevisiae. Sci Rep 8(1):5853
Wang X, Tsai JW, Imai JH, Lian WN, Vallee RB, Shi SH (2009) Asymmetric centrosome inheritance maintains neural progenitors in the neocortex. Nature 461(7266):947–955
Yamashita YM, Mahowald AP, Perlin JR, Fuller MT (2007) Asymmetric inheritance of mother versus daughter centrosome in stem cell division. Science 315(5811):518–521
Yeh E, Skibbens RV, Cheng JW, Salmon ED, Bloom K (1995) Spindle dynamics and cell cycle regulation of dynein in the budding yeast, Saccharomyces cerevisiae. J Cell Biol 130(3):687–700
Acknowledgements
R. F. researches are supported by Grants from PRIN (Progetti di Ricerca di Interesse Nazionale) and from the University of Milano Bicocca (FA). E. R. is supported by a fellowship from the Associazione Italiana per Ricerca sul Cancro (AIRC), Love Design Rif: 18196.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Kupiec.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Raspelli, E., Fraschini, R. Spindle pole power in health and disease. Curr Genet 65, 851–855 (2019). https://doi.org/10.1007/s00294-019-00941-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00294-019-00941-7