Abstract
Background
Diffuse astrocytic and oligodendroglial gliomas are the most common neuroepithelial tumors. Their classification is based on the integration of histological and molecular findings according to the classification of tumors of the central nervous system published by the World Health Organization (WHO) in 2016.
Objectives
This review describes the different entities and variants of diffuse gliomas and summarizes the current diagnostic criteria for these tumors.
Materials and methods
Based on the 2016 WHO classification and selected other publications, the histomolecular diagnostics of diffuse gliomas is presented and illustrated.
Results
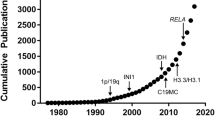
Diffuse gliomas are divided into isocitrate dehydrogenase (IDH)-mutant or IDH-wildtype gliomas by detection of mutations in the IDH1 or IDH2 genes. Among the IDH-mutant gliomas, oligodendroglial tumors are characterized by combined losses of chromosome arms 1p and 19q. Loss of nuclear expression of the ATRX protein is a marker of IDH- mutant astrocytic gliomas. Glioblastoma, IDH-wildtype, is the most common diffuse glioma. Diffuse and anaplastic astrocytic gliomas without IDH mutation should be further evaluated for molecular features of glioblastoma, IDH-wildtype. Diffuse gliomas in the thalamus, brainstem, or spinal cord carrying a histone 3 (H3)-K27M mutation are classified as diffuse midline gliomas, H3-K27M-mutant. By determining the IDH and 1p/19q status, oligoastrocytomas can be stratified into either astrocytic or oligodendroglial gliomas. Gliomatosis cerebri is no longer regarded as a distinct glioma entity.
Conclusions
Diffuse gliomas can today be classified accurately and reproducibly by means of histological, immunohistochemical, and molecular analyses.
Zusammenfassung
Hintergrund
Diffuse astrozytäre und oligodendrogliale Gliome sind die häufigsten neuroepithelialen Tumoren. Ihre diagnostische Einordnung erfolgt durch Integration histologischer und molekularer Befunde gemäß der 2016 von der Weltgesundheitsorganisation (WHO) herausgegebenen Klassifikation der Tumoren des zentralen Nervensystems.
Ziel der Arbeit
Die Arbeit beschreibt die verschiedenen Entitäten und Varianten diffuser Gliome und die diagnostischen Kriterien für diese Tumoren.
Material und Methoden
Basierend auf der WHO-Klassifikation 2016 und ausgewählten aktuellen Veröffentlichungen wird die histomolekulare Diagnostik diffuser Gliome erläutert und anhand eigener Befunde illustriert.
Ergebnisse
Diffuse Gliome werden durch Nachweis von Mutationen in den Isocitratdehydrogenase(IDH)-Genen 1 oder 2 in IDH-mutierte und IDH-Wildtyp-Gliome unterteilt. Unter den IDH-mutierten Gliomen zeigen oligodendrogliale Tumoren kombinierte Verluste der Chromosomenarme 1p und 19q. Ein Verlust der nukleären Expression des ATRX-Proteins spricht dagegen für ein IDH-mutiertes astrozytäres Gliom. Glioblastome (IDH-Wildtyp) sind insgesamt die häufigsten diffusen Gliome. Diffuse und anaplastische Astrozytome ohne IDH-Mutation sollten auf molekulare Merkmale eines Glioblastoms, IDH-Wildtyp überprüft werden. Diffuse Gliome im Thalamus, Hirnstamm oder Rückenmark, bei denen eine Histon-3(H3)-K27M-Mutation vorliegt, werden als diffuse Mittelliniengliome, H3-K27M-mutiert klassifiziert. Anhand des IDH- und 1p/19q-Status lassen sich Oligoastrozytome entweder als astrozytäre oder oligodendrogliale Gliome einordnen. Die Gliomatosis cerebri wird nicht mehr als eigene Entität betrachtet.
Diskussion
Mittels histologischer, immunhistochemischer und molekularpathologischer Untersuchungen lassen sich diffuse Gliome heutzutage präzise und reproduzierbar diagnostizieren.



Similar content being viewed by others
References
Barresi V, Lionti S, Valori L, Gallina G, Caffo M, Rossi S (2017) Dual-genotype diffuse low-grade Glioma. Is it really time to abandon Oligoastrocytoma as a distinct entity? J Neuropathol Exp Neurol 76(5):342–346. https://doi.org/10.1093/jnen/nlx024
van den Bent M, Gan HK, Lassman AB, Kumthekar P, Merrell R, Butowski N, Lwin Z, Mikkelsen T, Nabors LB, Papadopoulos KP, Penas-Prado M, Simes J, Wheeler H, Walbert T, Scott AM, Gomez E, Lee H‑J, Roberts-Rapp L, Xiong H, Bain E, Ansell PJ, Holen KD, Maag D, Reardon DA (2017) Efficacy of depatuxizumab mafodotin (ABT-414) monotherapy in patients with EGFR-amplified, recurrent glioblastoma: results from a multi-center, international study. Cancer Chemother Pharmacol 80(6):1209–1217. https://doi.org/10.1007/s00280-017-3451-1
Brat DJ, Aldape K, Colman H, Holland EC, Louis DN, Jenkins RB, Kleinschmidt-DeMasters BK, Perry A, Reifenberger G, Stupp R, von Deimling A, Weller M (2018) cIMPACT-NOW update 3: recommended diagnostic criteria for “Diffuse astrocytic glioma, IDH-wildtype, with molecular features of glioblastoma, WHO grade IV”. Acta Neuropathol. https://doi.org/10.1007/s00401-018-1913-0
Capper D, Jones DTW, Sill M et al (2018) DNA methylation-based classification of central nervous system tumours. Nature 555(7697):469–474. https://doi.org/10.1038/nature26000
Capper D, Stichel D, Sahm F, Jones DTW, Schrimpf D, Sill M, Schmid S, Hovestadt V, Reuss DE, Koelsche C, Reinhardt A, Wefers AK, Huang K, Sievers P, Ebrahimi A, Schöler A, Teichmann D, Koch A, Hänggi D, Unterberg A, Platten M, Wick W, Witt O, Milde T, Korshunov A, Pfister SM, von Deimling A (2018) Practical implementation of DNA methylation and copy-number-based CNS tumor diagnostics: the Heidelberg experience. Acta Neuropathol 136(2):181–210. https://doi.org/10.1007/s00401-018-1879-y
Ceccarelli M, Barthel FP, Malta TM et al (2016) Molecular profiling reveals biologically discrete subsets and pathways of progression in diffuse Glioma. Cell 164(3):550–563. https://doi.org/10.1016/j.cell.2015.12.028
Chen L, Voronovich Z, Clark K, Hands I, Mannas J, Walsh M, Nikiforova MN, Durbin EB, Weiss H, Horbinski C (2014) Predicting the likelihood of an isocitrate dehydrogenase 1 or 2 mutation in diagnoses of infiltrative Glioma. Neuro-oncology 16(11):1478–1483. https://doi.org/10.1093/neuonc/nou097
Cimino PJ, Zager M, McFerrin L, Wirsching H‑G, Bolouri H, Hentschel B, von Deimling A, Jones D, Reifenberger G, Weller M, Holland EC (2017) Multidimensional scaling of diffuse gliomas: application to the 2016 World Health Organization classification system with prognostically relevant molecular subtype discovery. Acta Neuropathol Commun 5(1):39. https://doi.org/10.1186/s40478-017-0443-7
von Deimling A, Ono T, Shirahata M, Louis DN (2018) Grading of diffuse astrocytic Gliomas: a review of studies before and after the advent of IDH testing. Semin Neurol 38(1):19–23. https://doi.org/10.1055/s-0038-1636430
Hartmann C, Meyer J, Balss J, Capper D, Mueller W, Christians A, Felsberg J, Wolter M, Mawrin C, Wick W, Weller M, Herold-Mende C, Unterberg A, Jeuken JWM, Wesseling P, Reifenberger G, von Deimling A (2009) Type and frequency of IDH1 and IDH2 mutations are related to astrocytic and oligodendroglial differentiation and age: a study of 1,010 diffuse Gliomas. Acta Neuropathol 118(4):469–474. https://doi.org/10.1007/s00401-009-0561-9
Herrlinger U, Jones DTW, Glas M, Hattingen E, Gramatzki D, Stuplich M, Felsberg J, Bähr O, Gielen GH, Simon M, Wiewrodt D, Schabet M, Hovestadt V, Capper D, Steinbach JP, von Deimling A, Lichter P, Pfister SM, Weller M, Reifenberger G (2016) Gliomatosis cerebri: no evidence for a separate brain tumor entity. Acta Neuropathol 131(2):309–319. https://doi.org/10.1007/s00401-015-1495-z
Herrlinger U, Tzaridis T, Mack F et al (2017) ACTR-58. Phase III trial of CCNU/temozolomide (TMZ) combination therapy vs. standard TMZ therapy for newly diagnosed MGMT-methylated glioblastoma patients: the CeTeT/NOA-09 trial. Neuro-oncology 19(Suppl 6):vi13–vi14. https://doi.org/10.1093/neuonc/nox168.049
Ida CM, Lambert SR, Rodriguez FJ, Voss JS, Mc Cann BE, Seys AR, Halling KC, Collins VP, Giannini C (2012) BRAF alterations are frequent in cerebellar low-grade astrocytomas with diffuse growth pattern. J Neuropathol Exp Neurol 71(7):631–639. https://doi.org/10.1097/NEN.0b013e31825c448a
Korshunov A, Chavez L, Sharma T, Ryzhova M, Schrimpf D, Stichel D, Capper D, Sturm D, Kool M, Habel A, Kleinschmidt-DeMasters BK, Rosenblum M, Absalyamova O, Golanov A, Lichter P, Pfister SM, Jones DTW, Perry A, von Deimling A (2018) Epithelioid glioblastomas stratify into established diagnostic subsets upon integrated molecular analysis. Brain Pathol 28(5):656–662. https://doi.org/10.1111/bpa.12566
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW (2016) The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131(6):803–820. https://doi.org/10.1007/s00401-016-1545-1
Louis DN, Aldape K, Brat DJ, Capper D, Ellison DW, Hawkins C, Paulus W, Perry A, Reifenberger G, Figarella-Branger D, Wesseling P, Batchelor TT, Cairncross JG, Pfister SM, Rutkowski S, Weller M, Wick W, von Deimling A (2017) Announcing cIMPACT-NOW: the Consortium to Inform Molecular and Practical Approaches to CNS Tumor Taxonomy. Acta Neuropathol 133(1):1–3. https://doi.org/10.1007/s00401-016-1646-x
Louis DN, Wesseling P, Paulus W, Giannini C, Batchelor TT, Cairncross JG, Capper D, Figarella-Branger D, Lopes MB, Wick W, van den Bent M (2018) cIMPACT-NOW update 1: Not Otherwise Specified (NOS) and Not Elsewhere Classified (NEC). Acta Neuropathol 135(3):481–484. https://doi.org/10.1007/s00401-018-1808-0
Louis DN, Giannini C, Capper D, Paulus W, Figarella-Branger D, Lopes MB, Batchelor TT, Cairncross JG, van den Bent M, Wick W, Wesseling P (2018) cIMPACT-NOW update 2: diagnostic clarifications for diffuse midline glioma, H3 K27M-mutant and diffuse astrocytoma/anaplastic astrocytoma, IDH-mutant. Acta Neuropathol 135(4):639–642. https://doi.org/10.1007/s00401-018-1826-y
Reifenberger G, Wirsching H‑G, Knobbe-Thomsen CB, Weller M (2017) Advances in the molecular genetics of Gliomas—implications for classification and therapy. Nat Rev Clin Oncol 14(7):434–452. https://doi.org/10.1038/nrclinonc.2016.204
Sahm F, Schrimpf D, Jones DTW, Meyer J, Kratz A, Reuss D, Capper D, Koelsche C, Korshunov A, Wiestler B, Buchhalter I, Milde T, Selt F, Sturm D, Kool M, Hummel M, Bewerunge-Hudler M, Mawrin C, Schüller U, Jungk C, Wick A, Witt O, Platten M, Herold-Mende C, Unterberg A, Pfister SM, Wick W, von Deimling A (2016) Next-generation sequencing in routine brain tumor diagnostics enables an integrated diagnosis and identifies actionable targets. Acta Neuropathol 131(6):903–910. https://doi.org/10.1007/s00401-015-1519-8
Schreck KC, Guajardo A, Lin DDM, Eberhart CG, Grossman SA (2018) Concurrent BRAF/MEK inhibitors in BRAF V600-mutant high-grade primary brain tumors. J Natl Compr Canc Netw 16(4):343–347. https://doi.org/10.6004/jnccn.2017.7052
Shirahata M, Ono T, Stichel D et al (2018) Novel, improved grading system(s) for IDH-mutant astrocytic Gliomas. Acta Neuropathol 136(1):153–166. https://doi.org/10.1007/s00401-018-1849-4
Stichel D, Ebrahimi A, Reuss D, Schrimpf D, Ono T, Shirahata M, Reifenberger G, Weller M, Hänggi D, Wick W, Herold-Mende C, Westphal M, Brandner S, Pfister SM, Capper D, Sahm F, von Deimling A (2018) Distribution of EGFR amplification, combined chromosome 7 gain and chromosome 10 loss, and TERT promoter mutation in brain tumors and their potential for the reclassification of IDHwt astrocytoma to glioblastoma. Acta Neuropathol 136(5):793–803. https://doi.org/10.1007/s00401-018-1905-0
Synhaeve NE, van den Bent MJ, French PJ, Dinjens WNM, Atmodimedjo PN, Kros JM, Verdijk R, Dirven CMF, Dubbink HJ (2018) Clinical evaluation of a dedicated next generation sequencing panel for routine glioma diagnostics. Acta Neuropathol Commun 6(1):126. https://doi.org/10.1186/s40478-018-0633-y
Weller M, van den Bent M, Tonn JC, Stupp R, Preusser M, Cohen-Jonathan-Moyal E, Henriksson R, Le Rhun E, Balana C, Chinot O, Bendszus M, Reijneveld JC, Dhermain F, French P, Marosi C, Watts C, Oberg I, Pilkington G, Baumert BG, Taphoorn MJB, Hegi M, Westphal M, Reifenberger G, Soffietti R, Wick W (2017) European Association for Neuro-Oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial Gliomas. Lancet Oncol 18(6):e315–e329. https://doi.org/10.1016/S1470-2045(17)30194-8
Wick W, Dettmer S, Berberich A, Kessler T, Karapanagiotou-Schenkel I, Wick A, Winkler F, Pfaff E, Brors B, Debus J, Unterberg A, Bendszus M, Herold-Mende C, Eisenmenger A, von Deimling A, Jones DTW, Pfister SM, Sahm F, Platten M (2018) N2M2 (NOA20) phase I/II trial of molecularly matched targeted therapies plus radiotherapy in patients with newly diagnosed non-MGMT hypermethylated glioblastoma. Neuro-oncology. https://doi.org/10.1093/neuonc/noy161
Zacher A, Kaulich K, Stepanow S, Wolter M, Kohrer K, Felsberg J, Malzkorn B, Reifenberger G (2016) Molecular diagnostics of gliomas using next generation sequencing of a glioma-tailored gene panel. Brain Pathol. https://doi.org/10.1111/bpa.12367
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
G. Reifenberger has received research funding from Roche and Merck as well as fees for lectures or consulting activities from AbbVie. B. Malzkorn declares that he has no conflicts of interest.
The tissue samples used for the histological images in Figs. 2 and 3 were retrieved from the archive of the Institute of Neuropathology at Heinrich Heine University Düsseldorf. The use of archival samples for research purposes was approved by the Ethics Commission of the Medical Faculty of the Heinrich Heine University Düsseldorf (Study No. 3562).
The supplement containing this article is not sponsored by industry.
Additional information
Editor
W. Feiden, Trier
Rights and permissions
About this article
Cite this article
Malzkorn, B., Reifenberger, G. Integrated diagnostics of diffuse astrocytic and oligodendroglial tumors. Pathologe 40 (Suppl 1), 9–17 (2019). https://doi.org/10.1007/s00292-019-0581-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00292-019-0581-8