Abstract
This study aimed to enhance compatibility and thermal stability between thermoplastic starch (TPS) and polylactic acid (PLA) by incorporating citric acid-grafted starch (CA–Starch) at different concentrations (1 and 5%) with varying degrees of substitution for starch derived from sweet potatoes and yams (DY). Blends were produced using a constant TPS/PLA weight ratio of 60:40, both with and without a compatibilizer. The blending process was carried out using a torque rheometer under two distinct conditions. The results exhibited reduced particle sizes in TPS60/PLA40/CA–Starch blends, as evidenced by SEM images displaying improved interaction and smoother surfaces. Significantly, one of the properties that experienced substantial improvement was the compatibility between TPS and PLA, as indicated by reduced phase segregation within the blends. This improvement manifested in enhanced surface morphology, smaller particle sizes, and greater homogeneity in the blends. Additionally, an enhancement in thermal properties, particularly thermal stability, was observed with higher CA content. This study underscores that the incorporation of CA–Starch not only enhances compatibility but also improves thermal stability in TPS60/PLA40 blends, offering potential for the development of biocompatible materials with superior performance.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The issue of petroleum-based plastic waste has surged to the forefront of environmental concerns, greatly impacting waste accumulation and pollution levels [1]. This problem primarily stems from the widespread use of short-lived plastics, notably in packaging and disposable products, characterized by their large volume and resistance to biodegradation or composting. In response, there has been a growing impetus to explore biodegradable materials derived from renewable natural polymers [2]. Among these alternatives, starch emerges as a promising candidate due to its abundance, ease of production, environmental friendliness, cost-effectiveness, and manageable properties [3,4,5].
Starch, a polysaccharide comprising linear amylose and branched amylopectin, exhibits semicrystalline properties owing to intermolecular and intramolecular hydrogen bonding in crystalline regions, resulting in a high melting temperature [6, 7]. However, its inherent moisture barrier deficiencies and susceptibility to water limit its direct application as a plastic material [8]. Consequently, modification becomes imperative to address these shortcomings and achieve desirable properties.
One prevalent modification method involves the addition of plasticizers like water, glycerol, or sorbitol to native starch, resulting in thermoplastic starch (TPS) [9]. Glycerol, for instance, has shown promise in enhancing barrier properties and thermal stability, although challenges such as high moisture absorption and low mechanical resistance persist [10,11,12,13]. A viable strategy to mitigate these limitations involves blending TPS with other polymers like polylactic acid (PLA), derived from natural resources like corn, starch, or sugar [14, 15]. PLA stands out for its excellent water barrier properties, resistance, biodegradability, and thermal stability, making it an ideal complement to TPS.
However, achieving a TPS/PLA blend with optimal properties hinges on improving the interfacial adhesion between hydrophilic starch granules and hydrophobic PLA molecules [16, 17]. Introducing a compatibilizer agent with functional groups capable of interacting with starch hydroxyl groups offers a viable solution to enhance phase interaction, thus improving mechanical, biodegradation, and barrier properties [18, 19]. Various compatibilization strategies have been explored, including chemical cross-linking, amphiphilic bridging, componential modification, and interfacial transitioning, albeit often involving complex blending processes or non-biological substances [20, 21].
Recent attention has turned toward citric acid as a starch cross-linking agent due to its biologically derived nature and safety profile [22]. The multicarboxylic structure of citric acid facilitates interaction with starch hydroxyl groups, thereby enhancing water resistance while being economically advantageous and environmentally benign compared to other cross-linking agents, such as epichlorohydrin [23, 24], maleic anhydride [25, 26], boric acid [27], malonic acid [28], glutaraldehyde [29], urea [30], tartaric acid, and succinic acid, which can be hazardous to human health and the environment [31, 32]. Hence, CA has been called “the green cross-linker” [33]. Studies have demonstrated improvements in plasticizing and barrier properties through starch cross-linked with citric acid [34,35,36,37].
However, to the best of our knowledge, there have been no studies that have focused on the preparation and characterization of Dioscorea rotundata (yam) and Ipomoea batatas (sweet potato) for the production of cross-linked thermoplastic starch with CA in blends with polylactic acid. This research not only introduces an innovative approach in utilizing these valuable natural resources but also stands out for its cost-effective and energy-efficient cross-linking method. This approach reflects a strong commitment to sustainable property enhancement. Furthermore, this research could pave the way for promising applications, such as the development of filaments for 3D printing, further expanding the potential of these blend in the field of technology and advanced manufacturing.
Materials and methods
Raw materials
The yam (DY) Dioscorea rotundata and sweet potato (SP) Ipomoea batatas tubers were collected from the Municipality of San Jacinto, department of Bolívar, in the Montes de María Mountains of the Caribbean region of Colombia. Starch was obtained from them according to the methodology proposed by Aristizábal and Johanna [38]. The glycerol used as TPS plasticizer was purchased from Sigma-Aldrich, USA, and the CA was provided by Merck, Germany. The polylactic acid (Luminy LX175, density \(1.24\;{\text{g}}/{\text{cm}}^{3}\)) was imported from Total Corbion PLA, the Netherlands, and purchased at Quimicoplasticos (Medellín—Colombia).
Extraction process of native starches
To extract native starch from DY and SP, the tubers were first washed to remove dirt. Next, the samples were peeled, washed, and cut into cubes of approximately 3 cm per side. The cubes were ground in a (Samurai) food processor for 5 min with deionized water until a suspension was obtained. This suspension was then filtered through a Nylon cloth (80 mesh), and the filtrate was allowed to settle for 24 h. The blend was then decanted to obtain two phases. The supernatant was removed, and the pellet was dried in a natural convection oven (E&Q) at 60 °C for 8 h. A ceramic mortar was used to reduce the particle size of starch to a fine powder, which was then packed in hermetically sealed polyethylene bags for later use. The determination of the amylose and amylopectin contents of the DY and SP starches extracted was reported in a previous study conducted by the research team [39].
Preparation of modified starch with citric acid (CA–Starch)
The DY and SP starches modified with CA were prepared as per the procedure described by Pornsuksomboon et al. [40]. 40 g of CA was dissolved in 100 mL of distilled water. The pH level of the solution was adjusted to ∼4 using 10 M NaOH. This blend was then combined with 100 g of dried DY or SP and stirred until a homogeneous suspension was obtained. The suspension was stored for 12 h at room temperature and dried at 60 °C for 10 h. The dried sample was washed 3 or 4 times with distilled water to remove unreacted CA and excess NaOH. Finally, the precipitate was dried in a natural convection oven (E&Q) at 105 °C for 1.5 h. The citric acid-modified diamond yam starch (CADY) and the citric acid-modified sweet potato starch (CASP) were stored in airtight bags in a desiccator until use.
Determination of the degree of substitution (DS)
The degree of substitution is related to the chemical modification and indicates the average number of CA substitutions per anhydroglucose unit (AGU) in the starch. It was determined by titration according to the method proposed by [41, 42] with minor modifications. Approximately 1.0 g of dried (CADY) or (CASP) was placed in a 250 mL flask and 50 mL of 75% ethanol in distilled water was added. The flask was shaken and heated at 50 °C for 30 min and then cooled to room temperature. Next, a standard 0.500 M aqueous sodium hydroxide solution (20 mL) was added. The flask was sealed and stirred using a magnetic agitator for 24 h. Excess alkali was back-titrated with 0.500 M HCl using phenolphthalein as indicator of the end point (color change from pink to clear). Next, a blank titration was performed using unmodified starch. The titration volumes of the blank and the sample are expressed in milliliters, and the weight of the sample is expressed in grams, according to Eq. (1):
The DS is defined as the average number of sites per glucose unit that a substituent group possesses. The DS was calculated based on Eq. (2) [43]:
where %CA represents the percentage by weight of substituent, 162 g/mol is the molecular weight of the unsubstituted AGU, and 192 g/mol is the molecular weight of the citric acid substituent. Each sample was analyzed in triplicates.
Preparation of CA–Starch/TPS blends
To prepare CA–Starch/TPS blends, native SP or DY starch and glycerol were mixed using a Hamilton beach 6,4650 mixer per a weight ratio of 60:40 for 5 min at room temperature (25–31 °C). To define the percentages by weight of CA, refer to [9, 20]. Given that previous studies have reported percentages ranging from 1 to 4%, this study implemented 1 and 5% of citric acid-modified starch (CASP) or (CADY) in the high-speed mixer. Subsequently, all components underwent thorough mixing for 5 min at room temperature.
Obtaining TPSDY/PLA/(CADY) and TPSSP/PLA/(CASP) blends
The corresponding amounts of SP or DY starch, plasticizing agent (glycerol), CA, and PLA were blended in a Thermo Scientific HAAKE Rheomix torque rheometer using roller-type rotors. To determine the behavior of the samples in the torque rheometer, a mass of 57 g was used for the tests and the fill factor value corresponded to 70%. The mixer was operated under two different processing conditions: at a rotor speed of 50 and 60 rpm, and at temperatures 150 and 180 °C. The components were continuously mixed for 10 and 15 min, respectively, according to the methodology described by Caicedo et al. [44]. The corresponding amounts of plasticizer and organic acid were added as presented in Tables 1 and 2.
The results obtained in the characterization of plasticized starches at different glycerol ratios of 60:40, 70:30, and 80:20, indicate that the best ratio for this study is 60:40 because it allowed us to obtain manageable samples. These results are consistent with the study by Chotipraon et al. [45], wherein a TPS-to-PLA weight ratio of 60:40 was used because it provided satisfactory properties at a reasonable cost. The mechanical properties of TPS/PLA blends decrease with high proportions of TPS, whereas a higher proportion of PLA increases costs and lowers degradation rates. Based on the above, we also established a ratio of 60:40, with the unique variation of cross-linking starch concentrations with CA, for which a simple-classification factorial design with four formulations was conducted based on the methodology reported by Rodrigo Ortega–Toro, Alex López–Córdoba et al. in their corresponding study [46]. As a control, TPS from sweet potatoes and yams was used. A blend of these polymers was prepared in the absence of CA–Starch, as well as two other blends with 1% or 5% CA–Starch.
Scanning electron microscopy (SEM)
Sample morphology was observed using a JEOL JSM 6060 SEM. The TPS/PLA/CA–Starch blends were coated with a gold layer to produce conductive patterns. Micrographs were taken through an accelerating voltage of 15 kV. SEM images were processed using ImageJ® software, a Java-based open-source program for image analysis and processing. Micrographs were calibrated to correlate image pixel dimensions with physical dimensions since image processing algorithms require a grayscale binary image to enable segmentation. Particle size analysis assumes a non-spherical particle being described using multiple length and width measurements. The reported value is an equivalent spherical diameter according to the methodology reported by Mazzoli and Favoni [47].
Thermogravimetric analysis (TGA)
The thermal stability of the samples was controlled using a SETSYS Evolution TGA. The weight of each sample was approximately 13 mg. Measurements were made at a heating rate of 10 °C/min starting at 25 °C till 600 °C. Each sample was kept in an inert atmosphere under a continuous flow of nitrogen at 100 mL/minute to avoid thermo-oxidative reactions (Table 3).
Fourier-transform infrared spectroscopy (FTIR)
Fourier-transform infrared spectroscopy was used to assess the chemical structure of the TPS60/PLA40/CA–Starch blends using an IRAffinity-1 spectrometer; Shimadzu FTIR absorbance analyses were performed using the KBr disk technique. Each sample was scanned 100 times, from 4000 to 400 cm−1 at a resolution of 4 cm−1.
Results and discussion
Determination of the citric acid degree of substitution by titration
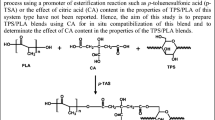
When CA is heated, it will dehydrate to produce an anhydride. This citric anhydride can react with starch to form citrate starch as shown in Fig. 1. The DS of a starch derivative is defined as the number OH groups per D-glucopyranosyl ring [48]. Since each ring has three hydroxyl (OH) groups, the maximum possible DS is 3. However, the primary OH group (C–6) is much more reactive than the two secondary OH groups (C–2 and C–3) due to steric hindrance [48, 49]. The DS and CA% values were 0.04 and 4.0%, respectively, for SP starch and 0.06% and 6.8%, respectively, for DY starch. The modified starch DS values obtained in this study are similar to those reported by Mei et al. [43], wherein the degree of substitution (SD) was 0.058. Nevertheless, DS variations could be caused by factors such as the botanical source of starch, the amylose and amylopectin content, the properties of starch granule size and structure, reagent concentration, reaction times, and temperature [49, 50]. It could be concluded that the DS values are consistent with those reported in the literature and that citric acid is an effective cross-linking reagent for the native starches used in this research.
Reaction chain of CA–Starch taken from [46]
Effect of CA percentage on rheometric properties of thermoplastic sweet potato (TPSSP/PLA/CASP) and yam (TPSDY/PLA/CADY) starch blends
The rheological behavior of the blends during processing is indicated in Table 4. After processing under conditions 1 and 2, all blend components were completely merged. This can be attributed to the PLA melting at the operating temperature since no non-plasticized pellets were observed after the test. The blend obtained under condition 1 exhibited a uniform color, which predominated in the entire mass removed from the rheometer, as shown in Fig. 2. The blend obtained under condition 2 presented a darker tint, possibly caused by the temperature as shown in Fig. 3.
The maximum torque ratio for TPSDY60/PLA40/CA–Starch samples denoted a significant decrease against the blank sample. As for the samples of TPSSP60/PLA40 in the presence of CA, an increase in the maximum torque was evident in tests 1 and 2. This may be due to the fact that the material opposes greater resistance to flow, thus increasing the torque and higher energy consumption in the process. These results suggest that the botanical source, the amylose/amylopectin content, and the particle size could influence the increased torque and energy consumption. In general, the minimum temperatures are maintained between 95 and 144 °C, and the energy of the process is proportional to the torque. The CA–Starch samples interact in the TPS-PLA matrix, reducing the mobility of the polymer chains, and increasing the required mechanical energy for blending the materials, as can be observed in Fig. 2b, c, e, f, as well as in Fig. 3b, c, e, f. According to the results, there is a better miscibility between the components as the CA–Starch ratio increases in the blend. Likewise, a better affinity of the functional groups of the TPS toward the polymeric chains of the PLA is evident since the maximum torque of the blend increases due to the fusion of the surface of the pellets merged to form small particles.
Similar results on the rheological properties were reported by Fourati et al. [51] who studied the effect of maleic anhydride (MA) and citric acid (CA) as compatibilizers in blends of poly (butylene adipate-co-terephthalate) (PBAT)/thermoplastic starch (TPS) in a weight ratio of 60:40. A significant evolution in the morphological and rheological properties of the blend was observed depending on the type of additives used. It was demonstrated that the type of compatibilizer controls the morphology and the development of the continuity of the TPS/PBAT blend. In the absence of compatibilizer, PBAT was the continuous phase, whereas TPS became continuous in the presence of MA and CA. These evolutions were explained by the change in the rheological properties in the molten state of TPS in the presence of compatibilizer and its ability to promote interfacial adhesion between TPS and PBAT phases. Additionally, the marked changes in the blend morphology were explained by the significant evolution of the viscosity and elasticity ratio between TPS and PBAT phases when MA and CA were added.
Effect of CA percentage on morphological, structural and thermal properties of thermoplastic sweet potato (TPSSP/PLA/CASP) and yam (TPSDY/PLA/CADY) starch blends
Morphological properties
In the assessment of polymeric blend systems, it is advisable to commence by elucidating the morphology, as it plays a pivotal role in determining other properties. The morphology of the TPS60/PLA40 blends with and without incorporation of CA–Starch was assessed using SEM at the following processing conditions: (1) 50 rpm, 150 °C and 10 min, and (2) 60 rpm, 180 °C and 15 min, as shown in Figs. 4 and 5. In these figures, as the content of CA cross-linked starch with CA increases, surface morphology changes. The TPS60/PLA40 samples for the two processing conditions and the two types of unmodified starches exhibited a heterogeneous morphology and a rough surface with agglomerations (Fig. 4a, d; Fig. 5a, d). This is due to the incompatibility and immiscibility between TPS and PLA polymers, which suggests phase separation. This may be caused by difference in polarity, thus generating a low affinity between these two polymers, since TPS is hydrophilic and PLA is hydrophobic [52]. Conversely, the blends containing CA–Starch presented a smoother surface (Fig. 4b, c, e, f; Fig. 5b, c, e, f). The increase in CA–Starch content in the blends is directly proportional to surface smoothness, which indicates more homogeneity and better interfacial adhesion between the phases generated by TPS and PLA due to the addition of CA–Starch and the specific processing conditions. The samples that yield smoother surfaces are found under the processing conditions at 60 rpm, 180 °C, and 15 min with incorporation of the two types of CA–Starch, which indicates that the cross-linking contributes to a better interfacial interaction because it acts as a plasticizer and compatibilizer for the interaction with hydroxyl groups of TPS and the polylactic acid chains. Table 5 lists average particle sizes from the image analysis of the SEM micrographs.
SEM micrographs of the blends at the followings conditions: 50 rpm, 150 °C, and 10 min. a TPSDY60/PLA40; b TPSDY60/PLA40/CADY1; c TPSDY60/PLA40/CADY5. SEM micrographs of the blends at the followings conditions: 50 rpm, 150 °C and 10 min d TPSSP60/PLA40; e TPSSP60/PLA40/CASP1; and f TPSSP60/PLA40/CASP5
The analysis of the SEM micrographs at 50 rpm, 150 °C, and 10 min for TPSDY60/PLA40 and TPSSP60/PLA40 (Fig. 4a, d) exhibited different surface morphologies. The results from the TPSDY60/PLA40 blends denoted a droplet-matrix structure between PLA and thermoplastic starch. This model is characteristic in immiscible blends wherein the minor phase (PLA) is distributed within the major phase (TPS matrix). Furthermore, we observed gaps at the interfaces between the TPS and PLA phases, confirming the low affinity of the two polymer phases.
On the other hand, the two types of CA–Starch samples present a smoother surface compared to the blends without compatibilizer (Fig. 4a and 4d). Still, we may observe agglomerations in the morphology of the DY starch blends. The SEM micrographs at 60 rpm, 180 °C, and 15 min yielded similar results to those obtained in the previous processing conditions for the TPSDY60/PLA40 and TPSSP60/PLA40 blends. Here, we observed heterogeneous blends, evidencing a phase separation. The micrographs (Fig. 4b, c, e, f; Fig. 5b, c, e, f) that contain CA–Starch showed good compatibility with a smoother surface as the cross-linked starch load increased, thus promoting an improvement in interfacial adhesion. These results suggest that better compatibility is obtained under these conditions than the 50 rpm, 150 °C, and 10 min conditions for CA–Starch blends; since here, the surface was smoother and the particle sizes were smaller, which can be attributed to higher compatibility. Marzieh et al. [17] obtained similar results, elucidating that in samples lacking maleic anhydride-based compatibilizer, thermoplastic starch particles did not uniformly disperse within the matrix, and some agglomerates were detected. This could be attributed to the contrasting hydrophilic nature of PLA and TPS phases. Conversely, in samples containing compatibilizers, spherical particles formed with improved dispersion, likely owing to enhanced interfacial adhesion between the two phases.
Fourier-transform infrared spectroscopy (FTIR)
FTIR spectroscopy of the TPS60/PLA40 and TPS60/PLA40/CA–Starch blends was performed to identify the functional groups and possible chemical interactions between the components, especially when the compatibilizer is present. Figures 6 and 7 illustrate the FTIR spectra for the DY and SP starch blends, respectively.
Figure 6 shows the spectra for the TPSDY60/PLA40 blends without and with compatibilizer at two processing conditions: (a) 50 rpm, 150 °C, and 10 min; (b) 60 rpm, 180 °C, and 15 min from the torque rheometer. The spectrum of both TPS blends denoted characteristic bands of DY starch at 3279 and 3300 cm−1 that can be attributed to the stretching vibrations of the –OH groups of amylose, amylopectin, glycerol, and adsorbed water during thermos plasticization. Other bands identified at 2939 and 2937 cm−1 were associated with the stretching of C–H groups. The peaks observed at 1646–1455 cm−1 (stretching with C–C and C–O–H) and 1076–996 cm−1 were associated with C–O stretching [46]. CA exhibits characteristic bands at 1076 cm−1 corresponding to COO stretching [20]. According to Reddy et al. [35] and Benjatham et al. [53], this peak change could be indicated between 1720 and 1740 cm−1, which is characteristic of an ester group. As shown, we did not find a 1720 cm−1 carboxylic band for any of the citric acid-modified starch blends. This could be due to the fact that the carbonyl group (–C=O) band stretches at 1750 cm−1 from PLA, and it is strong and close to the cross-linking band; therefore, it could be binding each other and affecting the observation of the COOH groups in the CA. The peaks at 1746 and 1748 cm−1 correspond to the stretching vibration of the –C=O carbonyl group. In addition, an absorption band is observed at 1160 and 1156 cm−1, which is associated with the stretching of the C–O group and it is characteristic PLA peaks. The results from the blends with citric acid cross-linked starch suggest displacement in the characteristic peaks at lower wave numbers. This indicates that the carbonyl groups also participate in the interaction between TPS and PLA, thus increasing the dispersion of the two phases [54]. The samples containing critic acid cross-linked starch exhibit an absorption band whose area increases with CA concentration: the absorption band at 1746 and 1748 cm−1. This band is characteristic of the stretching of carbonyl bonds. The presence of carbonyl bonds can be attributed to different causes: residual CA, presence of polylactic acid, and starch or glycerol esterification [55].
Figure 7 shows the FTIR spectra for the TPSSP60/PLA40 blends without and with compatibilizer at two processing conditions: (a) 50 rpm, 150 °C, and 10 min; (b) 60 rpm, 180 °C, and 15 min from the torque rheometer. All the spectra denoted the following characteristic bands: 3279 and 3295 cm−1, which corresponds to the stretching of the OH group belonging to starch, glycerol, and water. Similarly, the peak at 2980–2940 cm−1 represents –CH stretching. The 1644 cm−1 and 1646 cm−1 band is caused by the water absorbed by the starch molecules. We also identified other characteristic bands of starches at 1453 and 1454 cm−1 (C–H bending), 1205 cm−1 (C–OH stretching), 1078 and 1080 cm−1 (C–O bond stretching from –C–O–C), and 1030 and 1036 cm−1 (–CO stretching) [46, 56]. The PLA spectrum has an intense peak at the frequency 1743 and 1750 cm−1, which corresponds to the stretching of the bonds of the ester group (C=O). We observed characteristic traits of starch and PLA in the samples. However, we were unable to detect the characteristic peaks of CA because they were hindered by the vibrational peaks of the polymeric matrix. The samples containing citric acid cross-linked starches denote increases proportional to the percentage of CA in the C–O band at 1078 and 1080 cm−1. On the other hand, the band associated with hydroxyl groups (3300 cm−1) was observed to be broader and less intense in the case of materials containing CA, suggesting a reduced quantity of available OH groups due to the esterification reaction between the COOH groups of CA [57].
Thermogravimetric analysis (TGA)
The thermal stability of the TPS60/PLA40 blends without and with compatibilizer was assessed through a thermogravimetric analysis wherein we monitored weight loss due to thermal degradation caused by temperature changes. The thermogravimetric curves and the maximum degradation temperatures of the studied blends are shown in Figs. 8 and 9, and Tables 6 and 7, respectively.
Figure 8a and b presents the TGA and dTG curves for the blends with DY starch at 50 rpm, 150 °C, and 10 min, indicating weight loss due to the degradation of each material component as a function of temperature. The TPSDY60/PLA40 blends denoted a weight loss in three steps: at 50–173, 180–270, and 272–330 °C corresponding to evaporation of water, loss of glycerol, and TPS and PLA decomposition, respectively. On the derived thermogravimetric (dTG) curve of the TPSDY60/PLA40 blend, two degradation peaks are observed at 221.9 and 311.2 °C, which could indicate that the blend is immiscible and incompatible. Similar to TPSDY60/PLA40 blends, the TPSDY60/PLA40/CADY1 denoted a weight loss in three steps with two degradation peaks at 307.2 and 360.5 °C. Here, the largest mass loss corresponds to the degradation of the blend components. On the one hand, the TPS is between 300 and 320 °C, and on the other, the PLA is between 344 and 352 °C. The TPSDY60/PLA40/CADY5 sample with a larger citric acid load in the blend generated better thermal stability, which was observed in the TGA curve at a maximum peak of 327.6 °C. This indicates better compatibility between these two phases due to CA acting as an internal plasticizer of the polymeric matrix, thus causing the depolymerization of starch and improving the distribution of PLA in the matrix, favoring the mobility of polymeric chains, and decreasing the maximum degradation temperature.
Figure 8c and d presents the TGA and dTG curves for the blends containing SP starch at 50 rpm, 150 °C, and 10 min. The TPSSP60/PLA40 blends denoted a similar pattern as for the TPSDY60/PLA40 blends, exhibiting 3-step weight loss. The derived thermogravimetric (dTG) curve results for the TPSSP/PLA blend exhibit two drops at 220.3 and 311.2 °C. This gap may be due to the nature of the polymers. In the TPSSP60/PLA40/CASP1 and TPSSP60/PLA40/CASP5 samples, the maximum degradation temperatures are reported at 323.4 and 327.7 °C, respectively. The temperature values shifted to higher temperature values, which would indicate greater thermal stability.
The results obtained for the blends containing the two types of starches at the processing conditions of 50 rpm, 150 °C and 10 min suggest that the incorporation of the compatibilizing agent improves the interfacial adhesion between the components of the blend. This is indicated by the curve of the TPSDY60/PLA40/CADY5, TPSSP60/PLA40/CASP1, and TPSSP60/PLA40/CASP5 blending with a single peak in their DTG curves denoting good compatibility, whereas the TPSDY60/PLA40, TPSSP60/PLA40, and TPSDY60/PLA40/CADY1 blends with two peaks show some degree of incompatibility. Table 6 summarizes the initial degradation temperature (start) and the temperature at the maximum degradation rate (Peak) for the different blends. The results suggest that the incorporation of CA–Starch improves the compatibility between the two phases, thereby presenting a single peak on the dTG thermogram.
Figure 9a and b denotes the TGA and dTG performed for the blends containing SP starch at 60 rpm, 180 °C, and 15 min. The TPSSP60/PLA40 blend denoted a weight loss in three steps at 55–120 °C, 170–250 °C, and 260–350 °C, corresponding to water loss, glycerol volatilization, and PLA and starch decomposition, respectively, with a maximum degradation temperature at 326.8 °C. In the TPSSP60/PLA40/CASP1 and TPSSP60/PLA40/CASP5 samples, the maximum degradation temperatures are reported at 322.8 and 294.9 °C, respectively. The blends containing a compatibilizer denoted degradation temperatures that decreased as the CA loads increase, which indicates a certain degree of interaction within blend components, e.g., a certain degree of miscibility [58].
Figure 9c and d depicts the TGA and dTG performed for the blends containing DY at 60 rpm, 180 °C, and 15 min. The curves revealed several weight loss steps, the initial step between 50 and 180 °C corresponding to the loss of water and plasticizer volatilization. The next step is observed at 225–325 °C, and it is attributable to superimposed degradation of starch and PLA, since both polymers have similar degradation temperatures. Finally, in the third phase, oxidation of the partially decomposed polymer chains occurs at temperatures exceeding 330 °C. The compatible blends denoted a degradation temperature lower than 320.5 °C and 294.4 for TPSDY60/PLA40/CADY1 and TPSDY60/PLA40/CADY5, respectively. Hence, cross-linking with CA promotes thermal degradation at lower temperatures. The results suggest that under the processing conditions at 60 rpm, 180 °C and 15 min, a single maximum degradation peak may be reached for all the blends assessed, which would indicate that there is no phase separation between the two polymers, as observed in the results from Table 7. In addition, the degradation temperature shifted toward lower values because CA can act as a plasticizer, thus weakening the intermolecular bonds between the polymer chains, and decreasing the maximum degradation temperature. Similar results have been observed in starch-based materials cross-linked with citric acid as well as other cross-linking agents [36, 59].
Conclusions
A new material based on native thermoplastic starch (TPS) and polylactic acid (PLA), compatibilized with citric acid-grafted starch (CA–Starch) at different concentrations, was synthesized. Two processing conditions were tested to evaluate the morphological, structural, and thermal properties of the blends. Rheometric analysis was conducted under two conditions: (1) 50 rpm, 150 °C, 10 min and (2) 60 rpm, 180 °C, 15 min. The results indicated that cross-linking CA–Starch may improve compatibility within the TPS/PLA (60:40) phase by reducing size and creating a smoother surface, as observed in SEM images. FTIR analysis confirmed characteristic peaks of TPS and PLA, with variations in band intensity attributed to different starch types. Cross-linking with CA shifted peaks to lower wave numbers, indicating enhanced TPS60/PLA40 interaction and phase dispersion. Thermogravimetric analysis revealed that processing conditions influence thermal properties. At 50 rpm, 150 °C, 10 min, phase separation occurred, resulting in two degradation temperature peaks. Higher CA loading improved thermal stability. However, at 60 rpm, 180 °C, 15 min, blends with compatibilizer showed decreasing degradation temperatures with increased CA loads, suggesting improved miscibility.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article. In addition, the datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Przybytek A, Sienkiewicz M, Kucińska-Lipka J, Janik H (2018) Preparation and characterization of biodegradable and compostable PLA/TPS/ESO compositions. Ind Crops Prod 122:375–383. https://doi.org/10.1016/j.indcrop.2018.06.016
Ramakrishnan N, Sharma S, Gupta A, Alashwal BY (2018) Keratin based bioplastic film from chicken feathers and its characterization. Int J Biol Macromol 111:352–358. https://doi.org/10.1016/j.ijbiomac.2018.01.037
Othman SH, Majid NA, Tawakkal ISMA, Basha RK, Nordin N, Shapi’i RA (2019) Tapioca starch films reinforced with microcrystalline cellulose for potential food packaging application. Food Sci Technol 39(3):605–612. https://doi.org/10.1590/fst.36017
Wu Z, Huang Y, Xiao L, Lin D, Yang Y, Wang H, Yang Y, Wu D, Chen H, Zhang Q, Qin W, Pu S (2019) Physical properties and structural characterization of starch/polyvinyl alcohol/graphene oxide composite films. Int J Biol Macromol 123:569–575. https://doi.org/10.1016/j.ijbiomac.2018.11.071
Dai L, Wang X, Mao X, He L, Li C, Zhang J, Chen Y (2024) Recent advances in starch-based coatings for the postharvest preservation of fruits and vegetables. Carbohydr Polym 328:121736. https://doi.org/10.1016/j.carbpol.2023.121736
Du X, Jia J, Xu S, Zhou Y (2007) Molecular structure of starch from pueraria lobata (willd) ohwi relative to kuzu starch. Starch – Stärke. 59(12):609–613. https://doi.org/10.1002/star.200700604
Jullanun P, Yoksan R (2020) Morphological characteristics and properties of TPS/PLA/cassava pulp biocomposites. Polym Test 88:106522. https://doi.org/10.1016/j.polymertesting.2020.106522
Averous L, Boquillon N (2004) Biocomposites based on plasticized starch: thermal and mechanical behaviours. Carbohydr Polym 56:111–122. https://doi.org/10.1016/j.carbpol.2003.11.015
Ning W, Xingxiang Z, Na H, Jianming F (2010) Effects of water on the properties of thermoplastic starch poly(lactic acid) blend containing citric acid. J Thermoplast Compos Mater 23:19–34. https://doi.org/10.1177/0892705708096549
Altayan MM, Al Darouich T, Karabet F (2017) On the plasticization process of potato starch: preparation and characterization. Food Biophys 12(4):397–403. https://doi.org/10.1007/s11483-017-9495-2
Moro TMA, Ascheri JLR, Ortiz JAR, Carvalho CWP, Meléndez-Arévalo A (2017) Bioplastics of native starches reinforced with passion fruit peel. Food Bioproc Tech 10:1798–1808. https://doi.org/10.1007/s11947-017-1944-x
He Y, Wang X, Wu D, Gong Q, Qiu H, Liu Y, Wu T, Ma J, Gao J (2013) Biodegradable amylose films reinforced by graphene oxide and polyvinyl alcohol. Mater Chem Phys 142:1–11. https://doi.org/10.1016/j.matchemphys.2013.05.036
Dawam Abdu AH, Pudjirahar S, Karina M, Dwi Putri O, Fauziyyah RH (2019) Fabrication and characterization of sweet potato starch-based bioplastics plasticized with glycerol. J Biol Sci 19(1):57–64. https://doi.org/10.3923/jbs.2019.57.64
Andrew JJ, Dhakal HN (2021) Sustainable biobased composites for advanced applications: recent trends and future opportunities-A critical review. Comp Part C Open Access. https://doi.org/10.1016/j.jcomc.2021.100220
Trinh BM, Chang CC, Mekonnen TH (2021) Facile fabrication of thermoplastic starch/poly (lactic acid) multilayer films with superior gas and moisture barrier properties. Polymer (Guildf) 223:123679. https://doi.org/10.1016/J.POLYMER.2021.123679
Wu XS (2011) Effect of glycerin and starch crosslinking on molecular compatibility of biodegradable poly(lactic acid)-starch composites. J Polym Environ 19:912–917. https://doi.org/10.1007/s10924-011-0298-0
Akrami M, Ghasemi I, Azizi H, Karrabi M, Seyedabadi M (2016) A new approach in compatibilization of the poly(lactic acid)/thermoplastic starch (PLA/TPS) blends. Carbohydr Polym 144:254–262. https://doi.org/10.1016/j.carbpol.2016.02.035
Debiagi F, Mello LRPF, Mali S (2017) Thermoplastic starch-based blends: processing, structural, and final properties. Starch-based materials in food packaging: processing, characterization and applications. Elsevier Inc., Amsterdam, pp 153–186. https://doi.org/10.1016/B978-0-12-809439-6.00006-6
Volpe V, De Feo G, De Marco I, Pantani R (2018) Use of sunflower seed fried oil as an ecofriendly plasticizer for starch and application of this thermoplastic starch as a filler for PLA. Ind Crops Prod 122:545–552. https://doi.org/10.1016/J.INDCROP.2018.06.014
Sadeghi Ghari H, Nazockdast H (2022) Morphology development and mechanical properties of PLA/differently plasticized starch (TPS) binary blends in comparison with PLA/dynamically crosslinked “TPS+EVA” ternary blends. Polymer (Guildf) 245:124729. https://doi.org/10.1016/j.polymer.2022.124729
Koh JJ, Zhang X, He C (2018) Fully biodegradable Poly(lactic acid)/Starch blends: a review of toughening strategies. Int J Biol Macromol 109:99–113. https://doi.org/10.1016/j.ijbiomac.2017.12.048
Ghanbarzadeh B, Almasi H, Entezami AA (2011) Improving the barrier and mechanical properties of corn starch-based edible films: effect of citric acid and carboxymethyl cellulose. Ind Crops Prod 33:229–235. https://doi.org/10.1016/j.indcrop.2010.10.016
Garg S, Jana AK (2007) Studies on the properties and characteristics of starch-LDPE blend films using cross-linked, glycerol modified, cross-linked and glycerol modified starch. Eur Polym J 43:3976–3987. https://doi.org/10.1016/j.eurpolymj.2007.06.030
Jyothi AN, Moorthy SN, Rajasekharan KN (2006) Effect of cross-linking with epichlorohydrin on the properties of cassava (Manihot esculenta Crantz). Starch/Staerke 58:292–299. https://doi.org/10.1002/star.200500468
Raquez JM, Nabar Y, Srinivasan M, Shin BY, Narayan R, Dubois P (2008) Maleated thermoplastic starch by reactive extrusion. Carbohydr Polym 74:159–169. https://doi.org/10.1016/j.carbpol.2008.01.027
Olivato JB, Grossmann MVE, Yamashita F, Eiras D, Pessan LA (2012) Citric acid and maleic anhydride as compatibilizers in starch/poly(butylene adipate-co-terephthalate) blends by one-step reactive extrusion. Carbohydr Polym 87:2614–2618. https://doi.org/10.1016/j.carbpol.2011.11.035
Yin Y, Li J, Liu Y, Li Z (2005) Starch crosslinked with poly(vinyl alcohol) by boric acid. J Appl Polym Sci 96:1394–1397. https://doi.org/10.1002/app.21569
Ghosh Dastidar T, Netravali AN (2012) “Green” crosslinking of native starches with malonic acid and their properties. Carbohydr Polym 90(4):1620–1628. https://doi.org/10.1016/j.carbpol.2012.07.041
Gonenc I, Us F (2019) Effect of glutaraldehyde crosslinking on degree of substitution thermal, structural, and physicochemical properties of corn starch. Starch - Stärke 71:1800046. https://doi.org/10.1002/star.201800046
Majeed Z, Mansor N, Ajab Z, Man Z (2017) Lignin macromolecule’s implication in slowing the biodegradability of urea-crosslinked starch films applied as slow-release fertilizer. Starch - Stärke 69:1600362. https://doi.org/10.1002/star.201600362
Owi WT, Ong HL, Sam ST, Villagracia AR, Kuo Tsai C, Akil HM (2019) Unveiling the physicochemical properties of natural Citrus aurantifolia crosslinked tapioca starch/nanocellulose bionanocomposites. Ind Crops Prod 139:111548. https://doi.org/10.1016/j.indcrop.2019.111548
Xie X, Liu Q (2004) Development and physicochemical characterization of new resistant citrate starch from different corn starches. Starch - Stärke 56(8):364–370. https://doi.org/10.1002/star.200300261
Salihu R, Abd Razak SI, Ahmad Zawawi N, Rafiq Abdul Kadir M, Izzah Ismail N, Jusoh N, Riduan Mohamad M, Hasraf Mat Nayan N (2021) Citric acid: a green cross-linker of biomaterials for biomedical applications. Eur Polym J 146:110271. https://doi.org/10.1016/j.eurpolymj.2021.110271
Da Róz AL, Zambon MD, Curvelo AAS, Carvalho AJF (2011) Thermoplastic starch modified during melt processing with organic acids: the effect of molar mass on thermal and mechanical properties. Ind Crops Prod 33:152–157. https://doi.org/10.1016/j.indcrop.2010.09.015
Reddy N, Yang Y (2010) Citric acid cross-linking of starch films. Food Chem 118:702–711. https://doi.org/10.1016/j.foodchem.2009.05.050
Wu H, Lei Y, Lu J, Zhu R, Xiao D, Jiao C, Xia R, Zhang Z, Shen G, Liu Y, Li S, Li M (2019) Effect of citric acid induced crosslinking on the structure and properties of potato starch/chitosan composite films. Food Hydrocoll 97:105208. https://doi.org/10.1016/j.foodhyd.2019.105208
González P, Medina C, Famá L, Goyanes S (2016) Biodegradable and non-retrogradable eco-films based on starch–glycerol with citric acid as crosslinking agent. Carbohydr polym 138:66–74
Aristizábal J, Sánchez T, Lorío DM (2007) Guía técnica para producción y análisis de almidón de yuca. Organización de las Naciones Unidas para la Agricultura y la Alimentación, Roma
Martinez Villadiego K, Arias Tapia MJ, Useche J, Ledesma Y, Leyton A (2020) Thermal and morphological characterization of native and plasticized starches of sweet potato (Ipomoea batatas) and Diamante Yam (Dioscorea rotundata). J Polym Environ 29(3):871–880. https://doi.org/10.1007/s10924-020-01898-2
Pornsuksomboon K, Holló BB, Szécsényi KM, Kaewtatip K (2016) Properties of baked foams from citric acid modified cassava starch and native cassava starch blends. Carbohydr Polym 136:107–112. https://doi.org/10.1016/j.carbpol.2015.09.019
Sodhi NS, Singh N (2005) Characteristics of acetylated starches prepared using starches separated from different rice cultivars. J Food Eng 70:117–127. https://doi.org/10.1016/j.jfoodeng.2004.09.018
Bello-Pérez LA, Agama-Acevedo E, Zamudio-Flores PB, Mendez-Montealvo G, Rodriguez-Ambriz SL (2010) Effect of low and high acetylation degree in the morphological, physicochemical and structural characteristics of barley starch. LWT Food Sci Technol 43:1434–1440. https://doi.org/10.1016/j.lwt.2010.04.003
Mei JQ, Zhou DN, Jin ZY, Xu XM, Chen HQ (2015) Effects of citric acid esterification on digestibility, structural and physicochemical properties of cassava starch. Food Chem 187:378–384. https://doi.org/10.1016/j.foodchem.2015.04.076
Caicedo C, Pulgarin HLC (2021) Study of the physical and mechanical properties of thermoplastic starch/poly(lactic acid) blends modified with acid agents. Processes 9(4):578. https://doi.org/10.3390/pr9040578
Chotiprayon P, Chaisawad B, Yoksan R (2020) Thermoplastic cassava starch/poly(lactic acid) blend reinforced with coir fibres. Int J Biol Macromol 156:960–968. https://doi.org/10.1016/j.ijbiomac.2020.04.121
Ortega-Toro R, López-Córdoba A, Avalos-Belmontes F (2021) Epoxidised sesame oil as a biobased coupling agent and plasticiser in polylactic acid/thermoplastic yam starch blends. Heliyon 7:e06176. https://doi.org/10.1016/j.heliyon.2021.e06176
Mazzoli A, Favoni O (2012) Particle size, size distribution and morphological evaluation of airborne dust particles of diverse woods by scanning electron microscopy and image processing program. Powder Technol 225:65–71. https://doi.org/10.1016/j.powtec.2012.03.033
Mathew S, Abraham TE (2007) Physico-chemical characterization of starch ferulates of different degrees of substitution. Food Chem 105:579–589. https://doi.org/10.1016/J.FOODCHEM.2007.04.032
Ghosh Dastidar T, Netravali AN (2012) ‘Green’ crosslinking of native starches with malonic acid and their properties. Carbohydr Polym 90(4):1620–1628. https://doi.org/10.1016/J.CARBPOL.2012.07.041
Colussi R, El Halal SLM, Pinto VZ, Bartz J, Gutkoski LC, E. da R. Zavareze, A.R.G. Dias, (2015) Acetylation of rice starch in an aqueous medium for use in food. LWT - Food Sci Technol 62(2):1076–1082. https://doi.org/10.1016/J.LWT.2015.01.053
Fourati Y, Tarrés Q, Mutjé P, Boufi S (2018) PBAT/thermoplastic starch blends: effect of compatibilizers on the rheological, mechanical and morphological properties. Carbohydr Polym 199:51–57. https://doi.org/10.1016/J.CARBPOL.2018.07.008
Ferri JM, Garcia-Garcia D, Sánchez-Nacher L, Fenollar O, Balart R (2016) The effect of maleinized linseed oil (MLO) on mechanical performance of poly(lactic acid)-thermoplastic starch (PLA-TPS) blends. Carbohydr Polym 147:60–68. https://doi.org/10.1016/j.carbpol.2016.03.082
Sukkaneewat B, Panrot T, Rojruthai P, Wongpreedee T, Prapruddivongs C (2022) Plasticizing effects from citric acid/palm oil combinations for sorbitol-crosslinked starch foams. Mater Chem Phys 278:125732. https://doi.org/10.1016/J.MATCHEMPHYS.2022.125732
Wang N, Yu J, Chang PR, Ma X (2007) Influence of citric acid on the properties of glycerol-plasticized dry starch (DTPS) and DTPS/poly(lactic acid) blends. Starch/Staerke 59:409–417. https://doi.org/10.1002/star.200700617
Chabrat E, Abdillahi H, Rouilly A, Rigal L (2012) Influence of citric acid and water on thermoplastic wheat flour/poly(lactic acid) blends. I Thermal, mechanical and morphological properties. Ind Crops Prod 37(1):238–246. https://doi.org/10.1016/J.INDCROP.2011.11.034
Collazo-Bigliardi S, Ortega-Toro R, Chiralt A (2019) Using grafted poly(ε-caprolactone) for the compatibilization of thermoplastic starch-polylactic acid blends. React Funct Polym 142:25–35. https://doi.org/10.1016/J.REACTFUNCTPOLYM.2019.05.013
Seligra PG, Medina Jaramillo C, Famá L, Goyanes S (2016) Biodegradable and non-retrogradable eco-films based on starch-glycerol with citric acid as crosslinking agent. Carbohydr Polym 138:66–74. https://doi.org/10.1016/j.carbpol.2015.11.041
Bulatović VO, Mandić V, Kučić Grgić D, Ivančić A (2021) Biodegradable polymer blends based on thermoplastic starch. J Polym Environ 29:492–508. https://doi.org/10.1007/S10924-020-01874-W/FIGURES/12
Fonseca-García A, Osorio BH, Aguirre-Loredo RY, Calambas HL, Caicedo C (2022) Miscibility study of thermoplastic starch/polylactic acid blends: thermal and superficial properties. Carbohydr Polym 293:119744. https://doi.org/10.1016/j.carbpol.2022.119744
Acknowledgements
KMV and MJAT would like to express their gratitude to the Universidad Tecnológica de Bolívar for their support in facilitating the development of this research.
Funding
Open Access funding provided by Colombia Consortium. Internal Call 2019 funded by Universidad Tecnologica de Bolivar, entitled “Obtaining Biodegradable Polymers from Agricultural Products of the Montes de María Region” with ID C2018P024. Universidad Tecnológica de Bolívar, C2018P024, C2018P024
Author information
Authors and Affiliations
Contributions
KMV contributed to research, formal analysis, visualization, and writing—original draft. MJAT was involved in conceptualization, investigation, supervision, and writing—original draft. KMV, MJAT, and AFJ contributed to writing—reviewing and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Martínez-Villadiego, K., Arias-Tapia, M.J. & Jaramillo, A.F. Improving thermal stability of starches native cross-linked with citric acid as a compatibilizer for thermoplastic starch/polylactic acid blends. Polym. Bull. (2024). https://doi.org/10.1007/s00289-024-05315-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00289-024-05315-9