Abstract
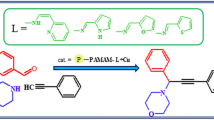
Porous polymer beads with grafted poly(tertiary amine) were prepared by grafting triethylenetetramine to porous crosslinked poly(methyl acrylate) beads via ester amidation reaction, followed by Eschweiler–Clarke methylation reaction using formaldehyde and formic acid to converting the primary and secondary amine groups to tertiary amines in the beads. The tertiary amine groups in the beads were protonated with hydrochloric acid, hydrobromic acid or hydriodic acid, and the protonated tertiary amine group-containing beads showed high catalytic activity and high selectivity for the formation of propylene carbonate through cycloaddition of propylene oxide with CO2, while free tertiary amine group-containing beads exhibited almost no catalytic activity. The recyclability of the catalyst was studied, and slight loss of the activity was observed after five runs.








Similar content being viewed by others
References
Guo L, Lamb KJ, North M (2021) Recent developments in organocatalysed transformations of epoxides and carbon dioxide into cyclic carbonates. Green Chem 23(1):77–118
Climate change: How do we know? https://climate.nasa.gov/evidence/. Accessed 16 June 2020
IPCC (2013) Summary for policymakers. In: Stocker TF, Qin D, Plattner G-K, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM (eds) Climate change 2013: the physical science basis. Contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge, pp 1–30
Zahasky C, Krevor S (2020) Global geologic carbon storage requirements of climate change mitigation scenarios. Energy Environ Sci 13(16):1561–1567. https://doi.org/10.1039/D0EE00674B
Meylan FD, Moreau V, Erkman S (2015) CO2 utilization in the perspective of industrial ecology an overview. J CO2 Util 12:101–108
Burkart MD, Hazari N, Tway CL, Zeitler EL (2019) Opportunities and challenges for catalysis in carbon dioxide utilization. ACS Catal 9(9):7937–7956. https://doi.org/10.1021/acscatal.9b02113
Dabral S, Schaub T (2019) The use of carbon dioxide (CO2) as a building block in organic synthesis from an industrial perspective. Adv Synth Catal 361(2):223–246. https://doi.org/10.1002/adsc.201801215
Kar S, Goeppert A, Prakash GKS (2019) Integrated CO2 capture and conversion to formate and methanol: connecting two threads. Acc Chem Res 52(10):2892–2903. https://doi.org/10.1021/acs.accounts.9b00324
Rehman A, Saleem F, Javed F, Ikhlaq A, Ahmad SW, Harvey A (2021) Recent advances in the synthesis of cyclic carbonates via CO2 cycloaddition to epoxides. J Environ Chem Eng 9(2):105113
Sakakura T, Choi J-C, Yasuda H (2007) Transformation of carbon dioxide. Chem Rev 107(6):2365–2387. https://doi.org/10.1021/cr068357u
Song Q-W, Zhou Z-H, He L-N (2017) Efficient, selective and sustainable catalysis of carbon dioxide. Green Chem 19(16):3707–3728. https://doi.org/10.1039/C7GC00199A
Schäffner B, Schäffner F, Verevkin SP, Börner A (2010) Organic carbonates as solvents in synthesis and catalysis. Chem Rev 110(8):4554–4581
Vivek JP, Berry N, Papageorgiou G, Nichols RJ, Hardwick LJ (2016) Mechanistic insight into the superoxide induced ring opening in propylene carbonate based electrolytes using in situ surface-enhanced infrared spectroscopy. J Am Chem Soc 138(11):3745–3751. https://doi.org/10.1021/jacs.5b12494
Blattmann H, Fleischer M, Bähr M, Mülhaupt R (2014) Isocyanate- and phosgene-free routes to polyfunctional cyclic carbonates and green polyurethanes by fixation of carbon dioxide. Macromol Rapid Commun 35(14):1238–1254. https://doi.org/10.1002/marc.201400209
Rokicki G, Parzuchowski PG, Mazurek M (2015) Non-isocyanate polyurethanes: synthesis, properties, and applications. Polym Adv Technol 26(7):707–761. https://doi.org/10.1002/pat.3522
Guo W, Gónzalez-Fabra J, Bandeira NAG, Bo C, Kleij AW (2015) A metal-free synthesis of N-aryl carbamates under ambient conditions. Angew Chem Int Ed 54(40):11686–11690. https://doi.org/10.1002/anie.201504956
Hara Y, Onodera S, Kochi T, Kakiuchi F (2015) Catalytic formation of α-aryl ketones by C–H functionalization with cyclic alkenyl carbonates and one-pot synthesis of isocoumarins. Org Lett 17(19):4850–4853. https://doi.org/10.1021/acs.orglett.5b02414
North M, Pasquale R, Young C (2010) Synthesis of cyclic carbonates from epoxides and CO2. Green Chem 12(9):1514–1539. https://doi.org/10.1039/c0gc00065e
Kleij AW, North M, Urakawa A (2017) CO2 catalysis. Chem Sus Chem 10(6):1036–1038. https://doi.org/10.1002/cssc.201700218
Sun H, Zhang D (2007) Density functional theory study on the cycloaddition of carbon dioxide with propylene oxide catalyzed by alkylmethylimidazolium chlorine ionic liquids. J Phys Chem A 111(32):8036–8043. https://doi.org/10.1021/jp073873p
Ma J, Liu J, Zhang Z, Han B (2012) The catalytic mechanism of KI and the co-catalytic mechanism of hydroxyl substances for cycloaddition of CO2 with propylene oxide. Green Chem 14(9):2410–2420. https://doi.org/10.1039/c2gc35711a
Aida T, Inoue S (1983) Activation of carbon dioxide with aluminum porphyrin and reaction with epoxide. Studies on (tetraphenylporphinato)aluminum alkoxide having a long oxyalkylene chain as the alkoxide group. J Am Chem Soc 105(5):1304–1309. https://doi.org/10.1021/ja00343a038
Paddock RL, Nguyen ST (2001) Chemical CO2 fixation: Cr(III) salen complexes as highly efficient catalysts for the coupling of CO2 and epoxides. J Am Chem Soc 123(46):11498–11499. https://doi.org/10.1021/ja0164677
Emelyanov MA, Stoletova NV, Lisov AA, Medvedev MG, Smol’yakov AF, Maleev VI, Larionov VA (2021) An octahedral cobalt(III) complex based on cheap 1,2-phenylenediamine as a bifunctional metal-templated hydrogen bond donor catalyst for fixation of CO2 with epoxides under ambient conditions. Inorg Chem Front 8:3871–3884
Cho W, Shin MS, Hwang S, Kim H, Kim M, Kim JG, Kim Y (2016) Tertiary amines: a new class of highly efficient organocatalysts for CO2 fixations. J Ind Eng Chem 44:210–215. https://doi.org/10.1016/j.jiec.2016.09.015
Kumatabara Y, Okada M, Shirakawa S (2017) Triethylamine hydroiodide as a simple yet effective bifunctional catalyst for CO2 fixation reactions with epoxides under mild conditions. ACS Sustain Chem Eng 5(8):7295–7301. https://doi.org/10.1021/acssuschemeng.7b01535
Caló V, Nacci A, Monopoli A, Fanizzi A (2002) Cyclic carbonate formation from carbon dioxide and oxiranes in tetrabutylammonium halides as solvents and catalysts. Org Lett 4(15):2561–2563. https://doi.org/10.1021/ol026189w
Ema T, Fukuhara K, Sakai T, Ohbo M, Bai F-Q, Hasegawa J-Y (2015) Quaternary ammonium hydroxide as a metal-free and halogen-free catalyst for the synthesis of cyclic carbonates from epoxides and carbon dioxide. Catal Sci Technol 5(4):2314–2321. https://doi.org/10.1039/C5CY00020C
Peng J, Deng Y (2001) Cycloaddition of carbon dioxide to propylene oxide catalyzed by ionic liquids. New J Chem 25(4):639–641. https://doi.org/10.1039/b008923k
Sun J, Zhang S, Cheng W, Ren J (2008) Hydroxyl-functionalized ionic liquid: a novel efficient catalyst for chemical fixation of CO2 to cyclic carbonate. Tetra Lett 49(22):3588–3591. https://doi.org/10.1016/j.tetlet.2008.04.022
Anthofer MH, Wilhelm ME, Cokoja M, Drees M, Herrmann WA, Kuhn FE (2015) Hydroxy-functionalized imidazolium bromides as catalysts for the cycloaddition of CO2 and epoxides to cyclic carbonates. ChemCatChem 7(1):94–98. https://doi.org/10.1002/cctc.201402754
Qiu M, Li J, Wu H, Huang Y, Guo H, Gao D, Shi L, Yi Q (2023) One-pot non-covalent heterogenization and aromatization of poly(ionic liquids) for metal-/cocatalyst-free and atmospheric CO2 conversion. Appl Catal B: Environ 322:122125
Kihara N, Hara N, Endo T (1993) Catalytic activity of various salts in the reaction of 2,3-epoxypropyl phenyl ether and carbon dioxide under atmospheric pressure. J Org Chem 58(23):6198–6202. https://doi.org/10.1021/jo00075a011
Liang S, Liu H, Jiang T, Song J, Yang G, Han B (2011) Highly efficient synthesis of cyclic carbonates from CO2 and epoxides over cellulose/KI. Chem Commun 47(7):2131–2133. https://doi.org/10.1039/C0CC04829A
Zhang Y-Y, Chen L, Yin S-F, Luo S-L, Au C-T (2015) Low-cost polymer-supported quaternary ammonium salts as high-efficiency catalysts for cycloaddition of CO2 to epoxides. Chem Sus Chem 8:2031–2034
Alassmy YA, Pour ZA, Pescarmona PP (2020) Efficient and easily reusable metal-free heterogeneous catalyst beads for the conversion of CO2 into cyclic carbonates in the presence of water as hydrogen-bond donor. ACS Sustain Chem Eng 8(21):7993–8003. https://doi.org/10.1021/acssuschemeng.0c02265
Chang T, Yan X, Li Y, Hao Y, Fu X, Liu X, Panchal B, Qin S, Zhu Z (2022) Quaternary ammonium immobilized PAMAM as efficient catalysts for conversion of carbon dioxide. J CO2 Util 58:101913
Yin QR, Li XH, Yan XL, Zhang XJ, Qin SJ, Hao YJ, Li NN, Zhu Z, Liu XH, Chang T (2023) Optimization and kinetics modeling of CO2 fixation into cyclic carbonates using urea-functionalized ionic organic polymers under mild conditions. Mol Catal 550:113601
Xie Y, Zhang Z, Jiang T, He J, Han B, Wu T, Ding K (2007) CO2 cycloaddition reactions catalyzed by an ionic liquid grafted onto a highly cross-linked polymer matrix. Angew Chem Int Ed 46(38):7255–7257. https://doi.org/10.1002/anie.200701467
Ghazali-Esfahani S, Song H, Păunescu E, Bobbink FD, Liu H, Fei Z, Laurenczy G, Bagherzadeh M, Yan N, Dyson PJ (2013) Cycloaddition of CO2 to epoxides catalyzed by imidazolium-based polymeric ionic liquids. Green Chem 15(6):1584–1589. https://doi.org/10.1039/c3gc37085b
Wan Y-L, Wang L, Wen L (2022) Amide-functionalized organic cationic polymers toward enhanced catalytic performance for conversion of CO2 into cyclic carbonates. J CO2 Utili 64:102174. https://doi.org/10.1016/j.jcou.2022
Beyzavi MH, Klet RC, Tussupbayev S, Borycz J, Vermeulen N, Cramer C, Stoddart JF, Hupp JT, Farha OK (2014) A hafnium-based metal–organic framework as an efficient and multifunctional catalyst for facile CO2 fixation and regioselective and enantioretentive epoxide activation. J Am Chem Soc 136(45):15861–15864. https://doi.org/10.1021/ja508626n
Das S, Zhang J, Chamberlain TW, Clarkson GJ, Walton RI (2022) Nonredox CO2 fixation in solvent-free conditions using a lewis acid metal–organic framework constructed from a sustainably sourced ligand. Inorg Chem 61(46):18536–18544. https://doi.org/10.1021/acs.inorgchem.2c02749
Lee S-D, Kim B-M, Kim D-W, Kim M-I, Roshan KR, Kim M-K, Won Y-S, Park D-W (2014) Synthesis of cyclic carbonate from carbon dioxide and epoxides with polystyrene-supported quaternized ammonium salt catalysts. Appl Catal A: Gen 486:69–76. https://doi.org/10.1016/j.apcata.2014.08.029
Whiteoak CJ, Henseler AH, Ayats C, Kleij AW, Pericàs MA (2014) Conversion of oxiranes and CO2 to organic cyclic carbonates using a recyclable, bifunctional polystyrene-supported organocatalyst. Green Chem 16(3):1552–1559. https://doi.org/10.1039/c3gc41919c
Qi C, Ye J, Zeng W, Jiang H (2010) Polystyrene-supported amino acids as efficient catalyst for chemical fixation of carbon dioxide. Adv Synth Catal 352(11–12):1925–1933. https://doi.org/10.1002/adsc.201000261
Liu Y, Hu Y, Zhou J, Zhu Z, Zhang Z, Li Y, Wang L, Zhang J (2021) Polystyrene-supported novel imidazolium ionic liquids: highly efficient catalyst for the fixation of carbon dioxide under atmospheric pressure. Fuel 305:121495
Dutcher B, Fan M, Russell AG (2015) Amine-based CO2 capture technology development from the beginning of 2013: a review. ACS Appl Mater Interfaces 7(4):2137–2148. https://doi.org/10.1021/am507465f
Schaffer A, Brechtel K, Scheffknecht G (2012) Comparative study on differently concentrated aqueous solutions of MEA and TETA for CO2 capture from flue gases. Fuel 148:148–153
Muchan P, Narku-Tetteh J, Saiwan C, Idem R, Supap T (2017) Effect of number of amine groups in aqueous polyamine solution on carbon dioxide (CO2) capture activities. Sep Purif Technol 184:128–134. https://doi.org/10.1016/j.seppur.2017.04.031
Yuan M, Gao G, Hu X, Luo X, Huang Y, Jin B, Liang Z (2018) Premodified sepiolite functionalized with triethylenetetramine as an effective and inexpensive adsorbent for CO2 capture. Ind Eng Chem Res 57(18):6189–6200. https://doi.org/10.1021/acs.iecr.8b00348
Zhang W, Gao E, Li Y, Bernards MT, He Y, Shi Y (2019) CO2 capture with polyamine-based protic ionic liquid functionalized mesoporous silica. J CO2 Util 34:606–615. https://doi.org/10.1016/j.jcou.2019.08.012
Zhang S, Cui Q, Li Y, Niu W, Li X, Liu J, Peng H, Jiang L, Yan H (2022) Immobilization of penicillin g acylase on resin carriers with amino groups. Ion Exch Adsorp 38:415–425
Liu M, Li X, Liang L, Sun J (2016) Protonated triethanolamine as multi-hydrogen bond donors catalyst for efficient cycloaddition of CO2 to epoxides under mild and cocatalyst-free conditions. J CO2 Util 16:384–390. https://doi.org/10.1016/j.jcou.2016.10.004
Aoyagi N, Furusho Y, Endo T (2012) Remarkably efficient catalysts of amidine hydroiodides for the synthesis of cyclic carbonates from carbon dioxide and epoxides under mild conditions. Chem Lett 41(3):240–241. https://doi.org/10.1246/cl.2012.240
Li C, Liu F, Zhao T, Gu J, Chen P, Chen T (2021) Highly efficient CO2 fixation into cyclic carbonate by hydroxyl-functionalized protic ionic liquids at atmospheric pressure. Mol Catal 511:111756
Bento AP, Bickelhaupt FM (2008) Nucleophilicity and leaving-group ability in frontside and backside SN2 reactions. J Org Chem 73(18):7290–7299. https://doi.org/10.1021/jo801215z
Liu M, Wang X, Jiang Y, Sun J, Arai M (2019) Hydrogen bond activation strategy for cyclic carbonates synthesis from epoxides and CO2: current state-of-the art of catalyst development and reaction analysis. Catal Rev 61:214–269. https://doi.org/10.1080/01614940.2018.1550243
Cokoja M, Wilhelm ME, Anthofer MH, Herrmann WA, Kuhn FE (2015) Synthesis of cyclic carbonates from epoxides and carbon dioxide by using organocatalysts. Chem Sus Chem 8(15):2436–2454. https://doi.org/10.1002/cssc.201500161
Acknowledgements
This work was supported by PCSIRT (IRT1257).
Author information
Authors and Affiliations
Contributions
WN contributed to the conceptualization, methodology, validation, data curation, investigation and writing—original draft. ZY contributed to the methodology, data curation and validation. DC contributed to the methodology, data curation and validation. LZ contributed to the methodology, data curation and validation. WG contributed conceptualization, methodology, data curation and writing—review and editing. HY contributed to the conceptualization, methodology, data curation, supervision and writing—review and editing.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there are no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Niu, W., Yin, Z., Chen, D. et al. Porous polymer beads with grafted poly(tertiary amine) as catalysts for fixation of carbon dioxide into propylene carbonate. Polym. Bull. (2024). https://doi.org/10.1007/s00289-024-05281-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00289-024-05281-2