Abstract
Magnetic nanoparticles have gained significant attention as versatile adsorbents in various studies due to their easy application, simple synthesis, cost-effectiveness, and reusability. In this study, we synthesized magnetic poly(AMA-co-GMA) nanoparticles (MAG) and modified them using allyl methacrylate (AMA) and glycidyl methacrylate (GMA). The epoxy groups on the nanoparticles were opened to introduce –OH, –NH2, and –SH functional groups. The structural properties of the synthesized nanoparticles were characterized by FTIR, their morphology by SEM–EDS, TEM, BET, and XRD, their magnetic properties by VSM, and the thermal properties by the TGA analysis. MAG and the modified nanoparticles (MAG-OH, MAG-NH2, and MAG-SH) were then employed in batch adsorption experiments for heavy metal ions, including Cd2+, Cu2+, Zn2+, Ni2+, Pb2+, and Hg2+. The effect of pH, contact time, ion concentration, and temperature on adsorption was investigated, and kinetic, isotherm, and thermodynamic parameters were determined. Pb2+ and Hg2+ exhibited higher adsorption capacities among the tested heavy metal ions, prompting further investigation with MAG and MAG-SH nanoparticles. The experimental data suggested that the adsorption followed the second order kinetic and Langmuir isotherm models. Overall, the results highlight the excellent potential of the synthesized magnetic nanoparticles for effectively removing heavy metal ions from wastewater.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The improper utilization of natural water resources and urban expansion resulting from rapid industrialization and population growth have contaminated water with numerous toxic substances. Among these pollutants, heavy metal ions such as lead (Pb), cadmium (Cd), mercury (Hg), chromium (Cr), copper (Cu), nickel (Ni), silver (Ag), and zinc (Zn) pose serious threats to the environment and public health due to their toxicity, persistence, and carcinogenic properties [1,2,3,4]. Various industrial activities, including paint production, agricultural pesticide use, automobile, emissions, the paper industry, mining operations, chemical manufacturing, metal refineries, and fertilizer application, contribute to releasing heavy metals into the environment [5]. The ingestion and accumulation of heavy metals in the human body can lead to severe health issues, including tremors, intellectual disability, developmental delays, liver and kidney damage, lung cancer, and anemia [6, 7]. Therefore, the effective removal of heavy metals is of utmost importance for human health. Different methods, such as chemical precipitation, ion exchange, solvent extraction, and adsorption, have been employed [8,9,10,11]. Adsorption is an appealing technique for heavy metal removal due to its environmental compatibility, cost-effectiveness, high efficiency, ease of implementation, simplicity of design, and compatibility with various adsorbents. It finds widespread application in various industries [12, 13]. Commonly used adsorbents for toxic metal removal include zeolite, activated carbon, graphene oxide, cellulose, biomaterials, polymeric materials, and magnetic nanoparticles [14,15,16,17]. Magnetic nanoparticles, in particular, have gained significant attention as adsorbents in numerous studies due to their ease of use, simple synthesis, cost-effectiveness, and reusability [18]. Synthetic nanoparticles are highly regarded due to their efficient adsorption properties, large surface areas, and the ability to be modified with various functional groups. The presence of epoxy in GMA and olefin groups in AMA enables structural modifications with different functional groups such as amino, thiol, and hydroxyl, allowing the customization of adsorbents to specific requirements [19].
In this study, we synthesized iron oxide nanoparticles and modified them using AMA-GMA. The epoxy groups were opened, and the nanoparticles were derivatized with -OH, -NH2, and -SH groups. The synthesized MAG and modified MAG nanoparticles were comprehensively characterized using FTIR, SEM–EDS, TEM, VSM, XRD, BET, and TGA. The performance of the four modified nanoparticles in adsorbing heavy metal ions was investigated under different parameters, and the kinetics of the adsorption process was elucidated.
Experimental
Materials and methods
FeCl3·6H2O and FeSO4·7H2O were obtained from Carlo Erba, glycidyl methacrylate (≥ 97.0%) and allyl methacrylate (98%) were sourced from Fluka, polyvinyl alcohol (PVA) (average Mw 31,000–50,000, 87–89% hydrolyzed), NH3 (25% aqueous solution), toluene (≥ 99.5%), tetrahydrofuran (≥ 99.0%) (THF), LiOH (98%), and n-octylamine (99%) were obtained from Merck. Benzoyl peroxide (with 25% H2O) and 2-thiazoline-2-thiol (98%) were obtained from Sigma-Aldrich. All other chemicals used in the study were of analytical purity and were employed without additional purification.
Synthesis of MAG nanoparticle
The synthesis procedure was adapted from the study conducted by Bayramoğlu [20]. Initially, a three-necked flask was filled with a 200 mL solution of 0.1 M FeCl3·6H2O under an N2 atmosphere, and the temperature was raised to 70 °C. Subsequently, 10 mL of toluene and 10 mL of a 5% PVA-H2O solution were introduced into the flask. After being stirred for one hour, 3 mL of GMA, 3 mL of AMA, and 0.05 g of benzoyl peroxide were added. The resulting mixture was stirred at this temperature for two hours before being heated to 80 °C and stirred for an additional hour. After adding 2.5 g of FeSO4·7H2O and 50 mL of distilled water, the temperature was increased to 90 °C, and the mixture was stirred for thirty minutes. Subsequently, 20 mL of a saturated NH3 solution was incorporated, and the mixture was stirred for an additional hour at this temperature. Finally, the mixture was centrifugated, and the solid component was thoroughly washed with distilled water and ethanol before being dried.
Modifications of MAG nanoparticle
–OH group modification
The synthesis procedure utilized in this study was derived from the research conducted by Leroux [21]. A total of 1.7 g of MAG were introduced into a two-necked flask, and 50 mL of distilled water was added. The flask was refluxed for 24 h, after which the resulting mixture was decanted. The solid residue was then thoroughly rinsed using distilled water and ethanol and dried.
–NH2 group modification
The synthesis procedure was adapted from Gao's study [22]. 1.7 g of MAG was placed in a two-necked flask, and 35 mL of ethanol was added. Next, 17 mL of n-octylamine was introduced into the flask, and the resulting mixture was stirred for 24 h at room temperature. Afterward, the mixture was decanted, and the solid was thoroughly rinsed with distilled water and ethanol before being dried.
–SH group modification
The synthesis procedure used in this study was adapted from Khan's research [23]. A total of 1.7 g of MAG was placed into a two-necked flask, and 35 mL of THF was added. Subsequently, 2.45 g of 2-thiazolin-2-thiol and 0.12 g of LiOH were added to the flask. The mixture was stirred at room temperature for 24 h. Afterward, the mixture was decanted, and the solid was washed with distilled water and ethanol, then dried.
Characterization techniques
The morphology and properties of the magnetic nanoparticles were elucidated using scanning electron microscopy coupled with X-ray energy-dispersive spectrometry (SEM–EDX), X-ray diffraction (XRD), vibrating sample measurement (VSM), thermogravimetry and Fourier transform infrared spectroscopy (FTIR), and Brunauer–Emmett–Teller (BET). FTIR analysis was performed using a Bruker platinum ATR-IR spectrometer in the range of 400–4000 cm−1, with a resolution of 4 cm−1. SEM–EDX analysis was carried out using a Quanta 400F field emission SEM device (FEI, USA) spectrometer. TGA measurement of the magnetic nanoparticles was performed using a TGA instrument (PerkinElmer Pyris). Thermogram was obtained in the range of 25–900 °C at 10 °C/min heating rate under N2 gas. XRD analysis was performed by Rigaku Ultima IV diffractometer with Cu Kα radiation (40 kV, 40 mA) in the range of 10°–90° at a scanning rate of 0.02° min−1. The magnetic property of magnetic nanoparticle was characterized at ± 1.0 T by VSM (Cryogenic Limited PPMS). The surface area and average pore size measurements of the MAG and MAG-SH nanoparticles were determined under nitrogen adsorption–desorption conditions, employing the BET equation (AUTOSORB-6B). The BET isotherm and the Barrett-Joyner-Halenda (BJH) method were utilized to determine specific surface area, pore volume, and pore size distribution.
Adsorption studies
MAG and the modified nanoparticles were utilized in the batch adsorption of Cd2+, Cu2+, Zn2+, Ni2+, Pb2+, and Hg2+ heavy metal ions. The effects of solution pH, initial ion concentration, temperature, and time were investigated. For this purpose, the pH values were adjusted to a range of 3.0–6.0, the initial concentration of ions ranged from 5 to 250 ppm, the temperature was set between 10 and 40 °C, the duration spanned from 155 to 120 min, and a total volume of 25 mL was prepared in Erlenmeyer flask. Adsorption studies were conducted at 25 °C and 150 rpm by adding 0.050 g of nanoparticles to the adsorption solution. The solution pH was adjusted using 0.1 M HCl and NaOH. The amount of adsorbed heavy metal ions was determined using ICP-OES. The adsorption per unit mass, qe (mg/g), which represents the amount of adsorbed substance at equilibrium, will be calculated using the following equation.
In this context, the initial and equilibrium concentrations of ions in solution will be denoted as parts per million (ppm), equivalent to milligrams per liter (mg/L). The variable V symbolizes the volume of the solution measured in liters, while M represents the mass of the adsorbent nanoparticles expressed in grams (Fig. 1).
Results and discussion
Characterization techniques
FTIR analysis
The FTIR spectra were obtained using the attenuated total reflectance (ATR) technique within the range of 4000–400 cm−1 (Fig. 2). In the FTIR spectrum of MAG nanoparticles, a peak corresponding to the Fe–O bond, characteristic of Fe3O4, is observed at 566 cm−1.[24] Moreover, a peak at 930 cm−1 cans be attributed to the epoxy group of GMA [25], while a peak at 979 cm−1 represents the C-H vibration of both GMA and AMA groups. The peaks observed at 1242 cm−1 and 1720 cm−1 correspond to the –C=C– group in AMA and the C=O and –CH2– vibrations in both GMA and AMA groups, respectively. Furthermore, a peak at 2936 cm−1 signifies the –CH2– vibration [26], whereas a peak at 3192 cm−1 indicates the –OH group. A peak at 3435 cm−1 confirms the occurrence of MAG-OH modification [27]. Additionally, the –NH2 band at 1646 cm−1 for MAG-NH2 [28] and the -SH vibration at 2642 cm−1 for MAG-SH further validate these modifications [29].
Thermogravimetric analysis
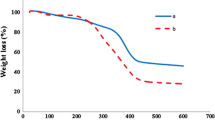
TGA was adopted to quantify the AMA-GMA coating values on the surface of Fe3O4 nanoparticles. The thermal degradation curve is given in Fig. 3. The thermogram of MAG nanoparticles coated with polymers reveals a mass reduction of approximately at 260 and 380 °C (Fig. 3A). The former was attributed to the decomposition of poly(GMA) while the latter to poly(AMA) [30, 31]. Previous studies have reported that uncoated iron oxide nanoparticles yield a mass loss of around 3% [32]. AMA-GMA polymers, on the other hand, are observed to undergo complete degradation at approximately 500 °C [26, 31]. The thermogram of MAG nanoparticles indicates a mass loss of approximately 11% at 600 °C. The maximum mass loss of MAG-SH between 580 °C and 720 °C is 20% at 670 °C and this mass loss is thought to be due to the degradation of bonded groups (Fig. 3B). This mass loss difference between coated and uncoated Fe3O4 nanoparticles was attributed to the presence of AMA-GMA polymers on the surface. The results suggest that MAG nanoparticles may have reached thermal stability.
Magnetic properties
The behavior of magnetic nanoparticles at a temperature of 298 K under varying magnetic field strengths was determined using a VSM, as depicted in Fig. 4. It was observed that the saturation magnetization value of the MAG nanoparticles was measured to be 41.6 Emu/g. It is worth noting that the thickness of the polymer layer surrounding the magnetic core and the properties of the polymer coating, such as molecular weight, can affect the saturation magnetization values of polymeric nanoparticles containing Fe3O4.[33, 34] In this study, the saturation magnetization value of the nanoparticles is considered sufficient for separating magnetic nanoparticles from solutions containing heavy metals using a simple magnet-assisted adsorption technique.
SEM–EDS and TEM analysis
The morphology of the magnetic polymer nanoparticles was examined using scanning electron microscopy and transmission electron microscopy. The analysis revealed that the nanoparticles exhibited a uniform spherical shape and tended to form clusters due to agglomeration. It was found that the size of the MAG nanoparticles was 68.37 ± 14.57 nm (Fig. 5A) and the size of the MAG-SH nanoparticles was 29.02 ± 7,65 nm (Fig. 5C) [35, 36]. This indicates that the -SH modification prevented agglomeration and resulted in the formation of smaller sized nanoparticles. The presence of Fe in the MAG nanoparticles was confirmed by the energy-dispersive X-ray spectroscopy (EDX) spectrum, indicating the successful incorporation of AMA-GMA copolymer nanoparticles (Fig. 5B).
BET analysis
The impact of nanoparticles on surface area, pore size, and pore volume was assessed using BET surface area and BJH pore size distribution methods (Fig. 6). The MAG nanoparticles had a surface area of 42.44 m2/g, a mean pore diameter of 3.12 nm, and a pore volume of 0.216 cm3/g. Similarly, the MAG-SH nanoparticles had a surface area of 75.39 m2/g, a mean pore diameter of 9.77 nm, and a pore volume of 0.21 cm.3/g. All particles exhibited comparable adsorption isotherms. The BET isotherms show that the synthesized particles exhibit a class IV isotherm, indicating that they are mesoporous materials. This is consistent with other published articles. [35] The results suggest that the nanoparticles are stable under functionalization and testing conditions. [36, 37]
X-ray diffraction (XRD) analysis
XRD was used to elucidate the amorphous and crystalline phases of MAG nanoparticles in the range of 10–90°. The X-ray diffraction spectrum of MAG nanoparticles is presented in Fig. 7. Upon comparison with previous studies, the spectrum exhibits distinct peaks at 2θ = 30.18°, 35.70°, 43.18°, 57.30°, and 63.06°, which can be assigned to the surface-centered cubic structure of Fe3O4.[38, 39]
Effect of pH on adsorption
The adsorption of metal ions is significantly affected by the pH value of the solution. Therefore, to determine the maximum adsorption capacities, the removal of heavy metals was investigated at various pH values. The adsorption capabilities of MAG, MAG-OH, MAG-NH2, and MAG-SH nanoparticles for Cd2+, Cu2+, Zn2+, Ni2+, Pb2+, and Hg2+ ions were evaluated across different pH values ranging from 3.0 to 6.0. The experimental conditions involved using 0.05 g of adsorbent, 50 ppm ion concentration, 25 °C temperature, and 150 rpm agitation speed for 120 min. The results, illustrated in Fig. 8, indicate that Cd2+, Cu2+, Zn2+, and Ni2+ ions exhibit minimal or negligible adsorption on all four polymers. On the other hand, the adsorption of Pb2+ and Hg2+ ions on MAG and MAG-SH increases as the pH value rises. Specifically, at pH 5.0, MAG demonstrates maximum adsorption capacities of 2.70 mg Pb/g and 7.20 mg Hg/g, while MAG-SH exhibits 21.28 mg Pb/g and 14.48 mg Hg/g capacities. Chen et al. determined pH 5 for Pb2+ in the adsorption of heavy metals using a polyaniline-type starch/glycidyl methacrylate copolymer. The maximum adsorption capacity of the copolymer was found to be 258 mg/g at an initial concentration of 5 mmol/L and 0.1 g adsorbent [40]. Bayramoğlu et al. [41] used an ammonia-functionalized magnetic terpolymer poly(glycidyl methacrylate-methyl methacrylate-ethylene glycol dimethacrylate) to remove Hg2+ions from aqueous solution under static conditions and in a magnetically stabilized fluidized bed (MFB) reactor. Optimum removal of Hg2+ions were observed at pH 5.5 with a maximum adsorption capacity of 124.8 mg/g.
Effect of time and temperature on adsorption and thermodynamics
To investigate the effect of time and temperature on adsorption and the adsorption behavior of Pb2+ and Hg2+ ions onto MAG and MAG-SH, batch experiments were conducted under specific conditions. The pH was set at 5.0, and the adsorption tests were carried out for durations ranging from 15 to 120 min and temperatures ranging from 10 to 40 °C. The initial ion concentration was maintained at 50 ppm, and the system was shaken at 150 rpm. The results are depicted in Fig. 9.
Figure 9 illustrates that the adsorption equilibrium of Pb2+ ion on MAG was achieved within 60 min, whereas on MAG-SH, equilibrium was reached within 30 min. For Hg2+ ion, an initial increase in adsorption was observed for both adsorbents until the 60-min mark, after which the adsorption capacity remained relatively constant. This indicates that the adsorbents attained their maximum saturation levels at approximately 60 min. Hee Kim and Gyu Lee synthesized porous poly(glycidyl methacrylate) (PMGA) functionalised with thiol groups and used it for HgII adsorption and found that the adsorption reached equilibrium in 4 h.[42] The effect of temperature on the adsorption capacity of the nanoparticles was examined. It was observed that the adsorption of Pb2+ ion on MAG increased from 2.34 mg/g to 6.02 mg/g as the temperature rose during the designated time frame (10, 20, 30, and 40 °C). In the case of MAG-SH, the adsorption capacity increased from 18.16 to 24.71 mg/g. Regarding Hg.2+ ion, the adsorption on MAG increased from 4.37 to 11.50 mg/g, and on MAG-SH, it increased from 12.55 to 17.91 mg/g. This temperature-dependent enhancement in adsorption suggests that the mobility of ions is augmented with increasing temperature, thereby facilitating stronger interactions with the active sites [11].
To gain deeper insights into the adsorption process, the thermodynamic parameters, including the standard Gibbs free energy change (ΔG°), standard enthalpy change (ΔH°), and standard entropy change (ΔS°), were calculated using the following equations [43]:
Here, ΔG° (kJ/mol) represents the change in standard free energy, ΔH° (kJ/mol) represents the change in standard enthalpy, ΔS° (J/mol·K) represents the change in standard entropy, and Kc (L/g) is the equilibrium constant.
By plotting in Kc against 1/T, the slope of the line determines ΔH°, and the intercept determines ΔS°. The results are presented in Table 1.
The standard enthalpy change (ΔH°) for the adsorption of Pb2+ and Hg2+ ions on MAG and MAG-SH was determined to be 28.715, 33.777, 81.836, and 23.623 kJ/mol, respectively. The positive value of ΔH° indicates that the adsorption is endothermic and chemical in nature. The positive value of ΔS° indicates an increase in randomness at the solid–liquid interface during Pb2+ and Hg2+ ions adsorption [11, 44].
Examining the calculated ΔG° values at different temperatures in the table, it can be observed that ΔG° for Pb2+ and Hg2+ ions on MAG is positive. These results indicate that the adsorption is not spontaneous and requires increased agitation rate and energy [45, 46]. On the other hand, the negative values of ΔG° obtained for MAG-SH indicate that the adsorption is spontaneous [47].
Effect of concentration on adsorption
The effect of concentration on equilibrium adsorption was investigated by varying the concentration within the range of 5–250 ppm. The experiments were conducted at pH 5.0, with a stirring speed of 150 rpm and an adsorption time of 120 min. The results are presented in Fig. 10. The initial concentration of metal ions is a crucial parameter that affects the mass transfer between the aqueous solution and the solid adsorbent in adsorption studies. Moreover, mass transfer significantly affects the adsorption capacities of the adsorbent molecules. The graph shows that the adsorption capacity increases with the increase of the concentration and reaches the equilibrium at about 100 ppm. The maximum adsorption capacities were determined to be 4.035 and 41.696 mg/g for Hg2+ and 4.195 and 38.996 mg/g for Pb2+ in the MAG and MAG-SH nanoparticles, respectively.
The augmentation in the initial metal ion concentration expedites mass transfer and the migration of ion molecules from the solution to the surface of nanoparticles [48, 49]. Consequently, nanoparticles adsorb larger ion molecules due to heightened interaction or collision. The adsorption capacity of nanoparticles attains equilibrium at around 100 ppm, as it is presumed that the surface of nanoparticles becomes saturated with ion molecules after a specific adsorption value, reducing available active sites on the adsorbent surface. Furthermore, electrostatic repulsion forces between the adsorbed ion molecules on the surface and the ion molecules in the solution make adsorption more challenging on unoccupied surfaces, necessitating additional time to reach equilibrium [50, 51].
Adsorption kinetics
Adsorption kinetics plays a crucial role in understanding the adsorption mechanism. Interpreting adsorption data concerning factors such as pH, time, temperature, and initial ion concentration provides valuable insights into adsorption. This study analyzed the adsorption data using first order and second order reaction kinetics. The first-order adsorption equation, as presented in equation, [52] was employed for this analysis.
In the provided equation, the qe and qt denote the quantities of ions adsorbed onto the nanoparticles at equilibrium and at time t (mg/g). The rate constant for first order adsorption is represented by k1 (1/min), while t represents the time measured in minutes. By plotting the natural logarithm of (qe−qt) against time, the slope of the resulting linear line yields the value of k1, and the intercept point provides the theoretical value of qe. The second order adsorption equation can be expressed as follows [53]:
In this equation, k2 (g/mg·min) represents the second order adsorption rate constant. The theoretical qe and the adsorption rate constant k2 can be determined by plotting t/qt against t, with the line slope representing qe and the intercept point indicating k2. The kinetic data obtained from the first- and second-order equations are presented in Table 2.
Upon analyzing the correlation coefficients of the linear lines obtained, it is evident that the adsorption of Pb2+ and Hg2+ ions on MAG and MAG-SH follows second-order reaction kinetics (R2 = 0.9243, 0.825, 0.9984, and 0.9943). Furthermore, the calculated qe values from the graphs of the second-order rate equation align well with the experimental values. [49, 54]
Adsorption isotherms
Adsorption isotherms are commonly employed to investigate the interactions between metal ions and adsorbents and to quantify their adsorption capacities [55]. In this study, the Langmuir and Freundlich isotherm models were employed to determine the adsorption capacities of Pb2+ and Hg2+ ions onto MAG and MAG-SH nanoparticles based on the collected adsorption data.
The Langmuir model is typically utilized to describe adsorption processes occurring on single-layer, homogeneous surfaces. In contrast, the Freundlich model is more suitable for explaining adsorption phenomena on heterogeneous surfaces with multiple layers [56].
The adsorption data were fitted to the Langmuir isotherm model using the following linear equation [57].
In the given equation, KL represents the Langmuir isotherm constant, which signifies the adsorbate's amount per unit mass of adsorbent (g/L). The Langmuir monolayer adsorption capacity, denoted as qm (mg/g), measures the adsorbent's maximum adsorption capacity. To determine these values, the slope and intercept of the linear Ce/qe−Ce curve were calculated using the obtained adsorption data. This curve was constructed to analyze the relationship between the equilibrium concentration (Ce) and the quantity of adsorbate adsorbed (qe) in accordance with the Langmuir model. Moreover, the Freundlich isotherm model was employed, and its linear form is expressed by the following equation [58].
In the provided equation, Kf represents the adsorption capacity [(mg/g)(L/mg)1/n], where n represents the adsorption intensity. A lnqe−lnCe graph was plotted to determine the Freundlich parameters, and the slopes and intercepts of the resulting linear curves were used. The Langmuir and Freundlich isotherm models were applied to the adsorption of Pb2+ and Hg2+ ions, and the data obtained are presented in Table 3.
The obtained high regression coefficients indicate that the Langmuir isotherm model is suitable for describing the adsorption of both ions. Additionally, the proximity of the obtained Langmuir monolayer adsorption capacities, qm, to the qe values further supports its appropriateness [59,60,61].
The magnitude of the n value, according to the Freundlich isotherm model, reflects the suitability of the process. A value of n < 1 suggests unsuitability for adsorption, n = 1 indicates linear adsorption and n > 1 indicates suitability for adsorption. Although the high n values obtained from the Freundlich isotherm model suggest its suitability, the low regression coefficients do not support this notion strongly. [49] These results indicate that the adsorption process exhibits a single-layer and homogeneous distribution.
Conclusions
In this study, magnetic poly(AMA-co-GMA) nanoparticles were synthesized and subsequently modified using AMA and GMA for surface functionalization. The epoxy groups present on the surface were further derivatized with –OH, –NH2, and –SH functional groups. To assess their structural and morphological characteristics, various characterization techniques, including FTIR, SEM–EDS, TEM, VSM, XRD, BET, and TGA were employed. These techniques have confirmed the modification of the nanoparticles, proved the imparted magnetic properties, demonstrated their spherical structure, and proved the surface-centered cubic structure. By BET analysis, the surface areas of MAG and MAG-SH nanoparticles were determined to be 42.44 and 75.39 m2/g, respectively. The synthesized magnetic nanoparticles and the modified nanoparticles obtained through surface functionalization were utilized for the adsorption of Pb2+ and Hg2+ heavy metal ions. The maximum adsorption capacities were determined to be 4.195, 4.035, 38.996, and 41.696 mg/g for Pb2+ and Hg2+ ions on MAG and MAG-SH, respectively. Furthermore, the adsorption behavior was found to conform to the second order kinetic model and the Langmuir isotherm model, as indicated by the data obtained from the experiments. These results highlight the promising potential of magnetic nanoparticles in effectively removing heavy metal ions from wastewater. Overall, this study demonstrates the successful synthesis and modification of magnetic poly(AMA-co-GMA) nanoparticles, followed by their efficient application for heavy metal ion adsorption. The comprehensive characterization techniques utilized provide valuable insights into the structural and morphological properties of the nanoparticles. The high adsorption capacities observed suggest the practical viability of employing these magnetic nanoparticles to treat heavy metal contaminated wastewater.
References
Gupta A, Jain R, Gupta DC (2015) Studies on uptake behavior of Hg(II) and Pb(II) by amine modified glycidyl methacrylate-styrene-N, N′-methylenebisacrylamide terpolymer. React Funct Polym 93:22–29. https://doi.org/10.1016/j.reactfunctpolym.2015.05.005
Zhu M, Wang X, Huang Y et al (2022) Synthesis of thiol-functionalized resin and its adsorption of heavy metal ions. J Appl Polym Sci. https://doi.org/10.1002/app.52976
Tyagi R, Jacob J (2020) Design and synthesis of water-soluble chelating polymeric materials for heavy metal ion sequestration from aqueous waste. React Funct Polym. https://doi.org/10.1016/j.reactfunctpolym.2020.104687
Driscoll CT, Mason RP, Chan HM et al (2013) Mercury as a global pollutant: sources, pathways, and effects. Environ Sci Technol 47:4967–4983
Başaran Kankiliç G, Metin AÜ, Aluç Y, Bozkaya O (2018) Bioremoval of mercury (II) from aqueous solutions by Phragmites australis : Kinetic and equilibrium studies. J Dispers Sci Technol 39:1790–1799. https://doi.org/10.1080/01932691.2018.1463243
Tekaya N, Saiapina O, Ben Ouada H et al (2013) Ultra-sensitive conductometric detection of heavy metals based on inhibition of alkaline phosphatase activity from Arthrospira platensis. Bioelectrochemistry 90:24–29. https://doi.org/10.1016/j.bioelechem.2012.10.001
Li Y, Bai P, Yan Y et al (2019) Removal of Zn2+, Pb2+, Cd2+, and Cu2+ from aqueous solution by synthetic clinoptilolite. Microporous Mesoporous Mater 273:203–211. https://doi.org/10.1016/j.micromeso.2018.07.010
Shrestha R, Ban S, Devkota S et al (2021) Technological trends in heavy metals removal from industrial wastewater: a review. J Environ Chem Eng. https://doi.org/10.1016/j.jece.2021.105688
Da̧browski A, Hubicki Z, Podkościelny P, Robens E (2004) Selective removal of the heavy metal ions from waters and industrial wastewaters by ion-exchange method. Chemosphere 56:91–106
El-Nadi YA (2017) Solvent extraction and its applications on ore processing and recovery of metals: classical approach. Sep Purif Rev 46:195–215
Moradi A, Najafi Moghadam P, Hasanzadeh R, Sillanpää M (2017) Chelating magnetic nanocomposite for the rapid removal of Pb(ii) ions from aqueous solutions: characterization, kinetic, isotherm and thermodynamic studies. RSC Adv 7:433–448. https://doi.org/10.1039/c6ra26356a
Peng W, Li H, Liu Y, Song S (2017) A review on heavy metal ions adsorption from water by graphene oxide and its composites. J Mol Liq 230:496–504
An FQ, Wu RY, Li M et al (2017) Adsorption of heavy metal ions by iminodiacetic acid functionalized D301 resin: Kinetics, isotherms and thermodynamics. React Funct Polym 118:42–50. https://doi.org/10.1016/j.reactfunctpolym.2017.07.005
Shirzadi H, Nezamzadeh-Ejhieh A (2017) An efficient modified zeolite for simultaneous removal of Pb(II) and Hg(II) from aqueous solution. J Mol Liq 230:221–229. https://doi.org/10.1016/j.molliq.2017.01.029
Yu JG, Yue BY, Wu XW et al (2016) Removal of mercury by adsorption: a review. Environ Sci Pollut Res 23:5056–5076. https://doi.org/10.1007/s11356-015-5880-x
Yang H, Bai T, Xue X et al (2015) A simple route to vinyl-functionalized hyperbranched polymers: self-condensing anionic copolymerization of allyl methacrylate and hydroxyethyl methacrylate. Polymer (Guildf) 72:63–68. https://doi.org/10.1016/j.polymer.2015.06.048
Liu S, Liu L, Su G et al (2022) Enhanced adsorption performance, separation, and recyclability of magnetic core-shell Fe3O4@PGMA-g-TETA-CSSNa microspheres for heavy metal removal. React Funct Polym. https://doi.org/10.1016/j.reactfunctpolym.2021.105127
Ahamad T, Naushad M, Alshehri SM (2020) Fabrication of magnetic polymeric resin for the removal of toxic metals from aqueous medium: kinetics and adsorption mechanisms. J Water Process Eng. https://doi.org/10.1016/j.jwpe.2020.101284
París R, Mosquera B, de la Fuente JL (2008) Atom transfer radical copolymerization of glycidyl methacrylate and allyl methacrylate, two functional monomers. Eur Polym J 44:2920–2926. https://doi.org/10.1016/j.eurpolymj.2008.06.031
Bayramoglu G, Tunali Y, Arica MY (2007) Immobilization of β-galactosidase onto magnetic poly(GMA-MMA) beads for hydrolysis of lactose in bed reactor. Catal Commun 8:1094–1101. https://doi.org/10.1016/j.catcom.2006.10.029
Gao H, Jones MC, Tewari P et al (2007) Star-shaped alkylated poly(glycerol methacrylate) reverse micelles: synthesis and evaluation of their solubilizing properties in dichloromethane. J Polym Sci A Polym Chem 45:2425–2435. https://doi.org/10.1002/pola.22004
Gao H, Elsabahy M, Giger EV et al (2010) Aminated linear and star-shape poly(glycerol methacrylate)s: synthesis and self-assembling properties. Biomacromol 11:889–895. https://doi.org/10.1021/bm901241k
De S, Khan A (2012) Efficient synthesis of multifunctional polymers via thiol-epoxy “lick” chemistry. Chem Commun 48:3130–3132. https://doi.org/10.1039/c2cc30434a
Singh S, Barick KC, Bahadur D (2011) Surface engineered magnetic nanoparticles for removal of toxic metal ions and bacterial pathogens. J Hazard Mater 192:1539–1547. https://doi.org/10.1016/j.jhazmat.2011.06.074
Jang J, Bae J, Ko S (2005) Synthesis and curing of poly(glycidyl methacrylate) nanoparticles. J Polym Sci A Polym Chem 43:2258–2265. https://doi.org/10.1002/pola.20706
Vardareli TK, Keskin S, Usanmaz A (2008) Synthesis and characterization of poly(allyl methacrylate) obtained by free radical initiator. J Macromol Sci Part A Pure Appl Chem 45:302–311. https://doi.org/10.1080/10601320701863783
Bukowska A, Bukowski W, Bester K, Flaga S (2015) Linkage of the PAMAM type dendrimer with the gel type resin based on glycidyl methacrylate terpolymer as a method of preparation of the polymer support for the recyclable palladium catalyst for Suzuki-Miyaura cross-coupling reactions. RSC Adv 5:49036–49044. https://doi.org/10.1039/C5RA04637H
Ko S, Jang J (2007) Protein immobilization on aminated Poly(glycidyl methacrylate) nanofibers as polymeric carriers. Biomacromol 8:1400–1403. https://doi.org/10.1021/bm070077g
Musa A, Kiskan B, Yagci Y (2014) Thiol-benzoxazine chemistry as a novel Thiol-X reaction for the synthesis of block copolymers. Polymer (Guildf) 55:5550–5556. https://doi.org/10.1016/j.polymer.2014.06.076
Zulfiqar S, Piracha A, Masud K (1996) The thermal degradation of poly(allyl methacrylate). Polym Degrad Stab 52:89–93. https://doi.org/10.1016/0141-3910(95)00237-5
Qassim MI, Khalill MMH, Hamed AA et al (2023) Synthesis and evaluation of a novel polycarbonate grafted poly (glycidyl methacrylate) resin for sorption of 131I. Radiat Phys Chem. https://doi.org/10.1016/j.radphyschem.2023.110774
Kayal S, Ramanujan RV (2010) Doxorubicin loaded PVA coated iron oxide nanoparticles for targeted drug delivery. Mater Sci Eng, C 30:484–490. https://doi.org/10.1016/j.msec.2010.01.006
Goya GF, Berquó TS, Fonseca FC, Morales MP (2003) Static and dynamic magnetic properties of spherical magnetite nanoparticles. J Appl Phys 94:3520–3528. https://doi.org/10.1063/1.1599959
Pourjavadi A, Kohestanian M, Keshavarzi N (2020) Immobilization of Au nanoparticles on poly(glycidyl methacrylate)-functionalized magnetic nanoparticles for enhanced catalytic application in the reduction of nitroarenes and Suzuki reaction. Appl Organomet Chem. https://doi.org/10.1002/aoc.5828
ur Rahman A, Iqbal M, ur Rahman F et al (2012) Synthesis and characterization of reactive macroporous poly(glycidyl methacrylate-triallyl isocyanurate-ethylene glycol dimethacrylate) microspheres by suspension polymerization: Effect of synthesis variables on surface area and porosity. J Appl Polym Sci 124:915–926. https://doi.org/10.1002/app.35026
Misra P, Chitanda JM, Dalai AK, Adjaye J (2015) Immobilization of fluorenone derived π-acceptors on poly (GMA-co-EGDMA) for the removal of refractory nitrogen species from bitumen derived gas oil. Fuel 145:100–108. https://doi.org/10.1016/j.fuel.2014.12.038
Xue P, Su W, Gu Y et al (2015) Hydrophilic porous magnetic poly(GMA-MBAA-NVP) composite microspheres containing oxirane groups: an efficient carrier for immobilizing penicillin G acylase. J Magn Magn Mater 378:306–312. https://doi.org/10.1016/j.jmmm.2014.11.048
Xu J, Ju C, Sheng J et al (2013) Synthesis and characterization of magnetic nanoparticles and its application in lipase immobilization. Bull Korean Chem Soc 34:2408–2412. https://doi.org/10.5012/bkcs.2013.34.8.2408
Ma M, Yang Y, Liao D et al (2019) Synthesis, characterization and catalytic performance of core-shell structure magnetic Fe3O4/P(GMA-EGDMA)-NH2/HPG-COOH-Pd catalyst. Appl Organomet Chem. https://doi.org/10.1002/aoc.4708
Chen Y, Zhao W, Wang H et al (2018) A novel polyamine-type starch/glycidyl methacrylate copolymer for adsorption of Pb(II), Cu(II), Cd(II) and Cr(III) ions from aqueous solutions. R Soc Open Sci 5:180281. https://doi.org/10.1098/rsos.180281
Bayramoğlu G, Arica MY (2007) Kinetics of mercury ions removal from synthetic aqueous solutions using by novel magnetic p(GMA-MMA-EGDMA) beads. J Hazard Mater 144:449–457. https://doi.org/10.1016/j.jhazmat.2006.10.058
Kim HH, Lee TG (2017) Removal of mercury ions in a simulated wastewater using functionalized poly(glycidyl methacrylate). J Ind Eng Chem 47:446–450. https://doi.org/10.1016/j.jiec.2016.12.019
Thajeel AS (2013) Isotherm, kinetic and thermodynamic of adsorption of heavy metal ions onto local activated carbon. Aquat Sci Technol. https://doi.org/10.5296/ast.v1i2.3763
Ali RM, Hamad HA, Hussein MM, Malash GF (2016) Potential of using green adsorbent of heavy metal removal from aqueous solutions: adsorption kinetics, isotherm, thermodynamic, mechanism and economic analysis. Ecol Eng 91:317–332. https://doi.org/10.1016/j.ecoleng.2016.03.015
Brown J, Mercier L, Pinnavaia TJ (1999) Selective adsorption of Hg2+ by thiol-functionalized nanoporous silica. Chem Commun. https://doi.org/10.1039/a807249c
Shabzendedar S, Modarresi-Alam AR, Noroozifar M, Kerman K (2020) Core-shell nanocomposite of superparamagnetic Fe3O4 nanoparticles with poly(m-aminobenzenesulfonic acid) for polymer solar cells. Org Electron. https://doi.org/10.1016/j.orgel.2019.105462
Guo K, Larson SL, Ballard JH et al (2018) Novel magnetic nanocarbon and its adsorption of Hg and Pb from water. Water Air Soil Pollut. https://doi.org/10.1007/s11270-018-3770-9
Hayati B, Maleki A, Najafi F et al (2018) Heavy metal adsorption using PAMAM/CNT nanocomposite from aqueous solution in batch and continuous fixed bed systems. Chem Eng J 346:258–270. https://doi.org/10.1016/j.cej.2018.03.172
Li Z, Wang Z, Wang C et al (2019) Preparation of magnetic resin microspheres M-P(MMA-DVB-GMA) and the adsorption property to heavy metal ions. Appl Surf Sci 496:143708. https://doi.org/10.1016/j.apsusc.2019.143708
Kumar KY, Muralidhara HB, Nayaka YA et al (2013) Low-cost synthesis of metal oxide nanoparticles and their application in adsorption of commercial dye and heavy metal ion in aqueous solution. Powder Technol 246:125–136. https://doi.org/10.1016/j.powtec.2013.05.017
Xu C, Feng Y, Li H et al (2022) Adsorption of heavy metal ions by iron tailings: behavior, mechanism, evaluation and new perspectives. J Clean Prod 344:131065. https://doi.org/10.1016/j.jclepro.2022.131065
Pan S, Shen H, Xu Q et al (2012) Surface mercapto engineered magnetic Fe3O4 nanoadsorbent for the removal of mercury from aqueous solutions. J Colloid Interface Sci 365:204–212. https://doi.org/10.1016/j.jcis.2011.09.002
Wu FC, Tseng RL, Huang SC, Juang RS (2009) Characteristics of pseudo-second-order kinetic model for liquid-phase adsorption: a mini-review. Chem Eng J 151:1–9
Yuan S, Zhang J, Yang Z et al (2017) Click functionalization of poly(glycidyl methacrylate) microspheres with triazole-4-carboxylic acid for the effective adsorption of Pb( <scp>ii</scp> ) ions. New J Chem 41:6475–6488. https://doi.org/10.1039/C7NJ00797C
Ma J, Shen J, Wang C, Wei Y (2018) Preparation of dual-function chelating resin with high capacity and adjustable adsorption selectivity to variety of heavy metal ions. J Taiwan Inst Chem Eng 91:532–538. https://doi.org/10.1016/j.jtice.2018.05.038
Freundlich HMF (1907) Über die Adsorption in Lösungen. Zeitschrift für Physikalische Chemie 57U:385–470. https://doi.org/10.1515/zpch-1907-5723
Langmuir I (1916) The constitution and fundamental properties of solids and liquids. Part I. Solids. J Am Chem Soc 38:2221–2295. https://doi.org/10.1021/ja02268a002
Nounou MN, Nounou HN (2010) Multiscale estimation of the Freundlich adsorption isotherm. Qatar Int J Environ Sci Tech 7:509–518
Radi S, Tighadouini S, El Massaoudi M et al (2015) Thermodynamics and kinetics of heavy metals adsorption on silica particles chemically modified by conjugated β-ketoenol furan. J Chem Eng Data 60:2915–2925. https://doi.org/10.1021/acs.jced.5b00281
Li M, Li B, Pan Y et al (2018) An aminosalicylic acid-modified cellulose composite used for mercury (II) removal from single and quarternary aqueous solutions. ChemistrySelect 3:10096–10102. https://doi.org/10.1002/slct.201802254
Chen Y, He M, Chen B, Hu B (2021) Thiol-grafted magnetic polymer for preconcentration of Cd, Hg, Pb from environmental water followed by inductively coupled plasma mass spectrometry detection. Spectrochim Acta Part B At Spectrosc 177:106071. https://doi.org/10.1016/j.sab.2021.106071
Acknowledgements
This work was supported by Scientific Research Projects Coordination Unit of Kırıkkale University. Project number 2020/089.
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Erduran, N., Işılar, Ö. Novel magnetic poly(allyl methacrylate-co-glycidyl methacrylate) Fe3O4 nanoparticles: synthesis and evaluation as adsorbents for heavy metals. Polym. Bull. (2024). https://doi.org/10.1007/s00289-024-05183-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00289-024-05183-3