Abstract
The objective of this study was to optimize the fermentation parameters by using response surface methodology for increasing pullulan production of Aureobasidium pullulans AZ-6 in synthetic fermentation medium. The optimum conditions were determined as follows: the initial sucrose concentration (X1): 100 g/L; the initial peptone concentration (X2): 11.31 g/L; the initial pH (X3): 6.48; and the temperature (X4): 24.2 °C. In the optimum conditions, the maximum exopolysaccharide (EPS) concentration (Y1), the maximum pullulan concentration (Y2), the specific growth rate (Y3), the maximum specific pullulan formation rate (Y4), and the pullulan yield (Y5) were expected to be obtained as 37.078 and 35.372 g/L, 0.062 h−1, 0.021 [g pullulan/(g mo.h)], and 53.681%, respectively. The observed values of Y1, Y2, Y3, Y4, and Y5 were 36.950 g/L, 35.470 g/L, 0.064 h−1, 0.036 [g pullulan/(g mo.sa)], and 54.480%, respectively, as a result of validation experiments. EPS samples were characterized by Fourier-transform infrared spectroscopy and scanning electron microscope analyses. The optimization process caused more than a 50% increase in EPS and pullulan concentrations. This study showed that the color-variant A. pullulans AZ-6 strain could become a significant industrial strain if it is explored further in the future to produce pullulan on a larger scale.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pullulan is an extracellular water-soluble homopolysaccharide produced by a yeast-like fungus A. pullulans [1]. It is usually defined as α-(1 → 6) linked linear polymer of maltotriose subunits [2]. Some distinctive properties of pullulan, such as structure flexibility, enhanced water-solubility, and excellent film- and fiber-forming properties, are attributed to regular alternation of α-(1 → 4) and α-(1 → 6) linkages in the ratio of 2:1 [3]. All these properties have made pullulan a potential candidate for many applications in the food, pharmaceutical, cosmetic and biomedical industries [4]. However, despite these benefits, the industrial production of pullulan has been limited due to the cost of medium constituents [5].
Many efforts have been devoted to optimizing the fermentation conditions to improve the pullulan yield and decrease the process cost [6]. In this regard, the RSM, which is used to identify the impacts of multiple factors influencing responses by modifying them simultaneously and conducting a small number of tests, is highly effective [6,7,8,9]. RSM is a collection of mathematical and statistical techniques useful for modeling and analysis of problems [10]. Central composite rotatable design (CCRD) is a widely used statistical technique for determining the key factors out of a large number of fermentation parameters by carrying out a small number of experiments. Fermentation parameters effecting pullulan production include carbon and nitrogen sources in the fermentation medium and their initial concentrations, dissolved oxygen levels, initial pH of the medium, temperature, fermentation time, and fungal strain.
The aim of this research was to determine the A. pullulans strain producing the highest concentration of pullulan and to optimize the various parameters effecting this strain’s pullulan production. First, the strain capable of producing the highest concentration of pullulan was selected among the seven different A. pullulans strains. Then, the effects of various carbon source and nitrogen source combinations on the pullulan production of this selected strain were investigated. Finally, the effects of four different fermentation parameters (initial sucrose and peptone concentrations, initial pH and temperature) on five different dependent variables (maximum EPS and pullulan concentrations, specific growth rate, maximum specific pullulan formation rate, and pullulan yield) were determined following CCRD and optimum fermentation conditions were determined by using RSM.
This study is a first in the literature in terms of optimization of pullulan production of domestic A. pullulans AZ-6 strain in synthetic fermentation medium. In addition, the color-variant characteristic of the A. pullulans AZ-6 strain is essential for the absence of melanin during pullulan formation and for potential commercial applications of this strain.
Materials and methods
Aureobasidium pullulans strains and selection
Aureobasidium pullulans strains used in this study and their isolation sources are listed in Table 1. These microorganisms were provided from various culture collections in lyophilized form or as slant cultures. Among the strains, the domestic A. pullulans AZ-6 strain had been isolated from fresh Gemlik olives in our previous study [11]. Aureobasidium pullulans strains were kept at 4 °C on yeast extract malt extract (YM) slants consisting of (in g/L): yeast extract, 3; malt extract, 3; peptone, 5; glucose, 10; and agar, 15. Cultures stored in YM agar were activated in YM broth by maintaining consecutive transfers. For long-term preservation, cultures were stored in yeast extract peptone dextrose (YEPD) liquid medium containing 20% glycerine in a freezer at − 70 °C.
To determine the A. pullulans strain that could produce the highest concentration of pullulan, preliminary tests were conducted in a fermentation media including (g/L) sucrose; 50, (NH4)2SO4; 2.0, yeast extract; 3.0, K2HPO4; 5.0, MgSO4.7H2O; 0.2, and NaCl; 1.0. Domestic A. pullulans AZ-6 had the highest concentration of pullulan among the tested strains (data not shown). Aureobasidium pullulans AZ-6 was also determined as a color-variant strain that generates pink colonies (Fig. 1) and does not produce melanin (a dark brown pigment) during pullulan synthesis. The lack of melanin production during pullulan synthesis is a desirable trait. Due to its higher pullulan production and being color-variant strain, further fermentation experiments were carried out using A. pullulans AZ-6.
Preparation of inoculum
For inoculum preparation, A. pullulans strains were cultured on the YM agar slants at 28 °C for 2 days, and the cultures were inoculated into 250 mL cotton-plugged Erlenmeyer flasks containing 50 mL of sterile growth media containing (g/L) sucrose; 30, (NH4)2SO4; 2.0, yeast extract; 3.0, K2HPO4; 5.0, MgSO4.7H2O; 0.2 and NaCl; 1.0. The culture was grown at 28 °C in water bath shakers with a shaking rate of 100 strokes/min for 48 h.
Equipment and fermentation conditions
Fermentation experiments were conducted in batch systems in shaking water baths (Grant SS40-D (UK), Haake SWB-20 (Germany), and Nuve ST-402 (Turkey)) with manageable temperature and agitation speed, using 250- and 300-mL flasks with a working volume of 50–150 mL. Throughout the experiments, agitation rates were kept constant at 100 strokes/minute. In experiments, a sterile fermentation medium containing (g/L) sucrose; 50, (NH4)2SO4; 2.0, yeast extract; 3.0, K2HPO4; 5.0, MgSO4.7H2O; 0.2 and NaCl; 1.0 was used after sterilization in an autoclave at 121 °C for 15 min.
Determining the effects of different carbon sources and nitrogen source combinations on pullulan production of A. pullulans AZ-6
To determine the effects of the different carbon sources on pullulan production of A. pullulans AZ-6, glucose, fructose, sucrose, galactose, or maltose were used as a substrate. For this purpose, sucrose in the basal fermentation medium has been replaced with the carbon source to be tested, keeping the initial concentration constant at 50 g/L.
Experiments were carried out in 300-mL cotton-plugged Erlenmeyer flasks containing basal fermentation medium in 150 mL working volumes. Inoculations of 5% (v/v) were made in the fermentation media from the pre-prepared inoculum culture with an initial inoculation concentration of approximately 1 × 107 cells/mL. The cultural method was used to determine the initial inoculation concentration of A. pullulans AZ-6 in fermentation media. Fermentations were performed in agitated water baths at 28 °C and at a constant agitation rate of 100 strokes/min.
To determine the effects of different nitrogen sources on pullulan production of A. pullulans AZ-6, the fermentation media consisting of (g/L) sucrose, 50; K2HPO4, 5.0; MgSO4.7H2O, 0.2; NaCl, 1.0, and combinations of various nitrogen sources with different concentrations were used. Experiments were carried out by adding a total of six different organic or inorganic nitrogen sources to fermentation media in various combinations. In these experiments, the total nitrogen concentrations in the compositions of the fermentation media to be tested were kept constant to be equal (0.739 g/L) to those in the basal fermentation medium. The initial nitrogen concentration of each fermentation medium containing two different nitrogen sources (organic and inorganic) was 0.739 g/L. The different nitrogen source combinations used in the experiments and their initial concentrations in fermentation media are given in Table 2.
In the experiments where the effects of different carbon sources and nitrogen source combinations on the pullulan production of A. pullulans AZ-6 strain were investigated, 10 mL of culture was collected from the fermentation media approximately every 24 h, and the EPS, pullulan, biomass, and substrate concentrations in the samples were determined. Then, graphs showing the changes of the obtained data against time were drawn, and maximum EPS and pullulan concentrations, specific growth rate, substrate consumption rate, and specific pullulan formation rate of the A. pullulans AZ-6 strain were calculated for each fermentation medium. After the experiments, the carbon and nitrogen combination of the fermentation medium in which the A. pullulans AZ-6 strain produced pullulan at the highest concentration was determined, and this carbon and nitrogen source combination was used in the fermentation mediums in the later stages of the study.
Analytic assays
Determination of biomass concentration
Biomass concentration was calculated as dry cell weight (DCW, g/L). During the experiments, 10 mL of culture sample was taken from the fermentation media approximately every 24 h. The sample was centrifuged (Nuve, NF 200, Turkey) at 5000 rpm for 20 min and then the supernatant was separated. The pellet was dried at 80 °C until the constant weight [12]. Biomass concentration was expressed as g/L.
Determination of exopolysaccharide and pullulan concentrations
The EPS concentration was determined by the precipitation method with cold ethyl alcohol [13]. For this purpose, 10 mL supernatant obtained after centrifuging the culture sample was mixed with 2 volumes of 95% (v/v) cold ethanol and held at 4 °C for 24 h to precipitate the crude EPS. After vigorous stirring, crude pullulan was separated by centrifugation at 4100 rpm for 20 min. After removal of the residual ethanol, the precipitate was dried at 80 °C till constant weight. The recovered EPS was estimated as g/L.
Pullulan content in the EPS was determined by using an enzymatic method [13]. Pure pullulan, on complete hydrolysis with pullulanase, yields only maltotriose and maltotetraose and therefore the yield of these oligosaccharides from the exhaustive hydrolysis of the EPS can be used to estimate their pullulan content. Hence, pullulan-containing ethanol precipitates were dissolved in distilled water (20 mg/L) and 0.5 ml aliquots were added in tubes containing 0.4 mL phosphate citric acid buffer (pH 5.0) and exhaustively hydrolyzed to maltotriose units with 0.1 mL pullulanase (Promozyme D2, Sigma, USA) at 40 °C for 2 h. The contents of glucose equivalent of maltotriose units were determined spectrophotometrically at 540 nm wavelength using the dinitrosalicylic acid (DNS) method [14].
In the experiments, pure pullulan (Sigma, USA) sample was used as a standard, and all the procedures applied to the analyzed EPS sample were also applied to the pure pullulan. During the spectrophotometric measurements, two blanks (enzyme and substrate) were used. Buffer and EPS containing enzyme-free solution were used as the enzyme blank, and the sample-free phosphate-citric acid buffer solution was used as the substrate blank. The equivalent glucose concentration calculated for the EPS sample was proportioned to the equivalent glucose concentration obtained for the pure pullulan, and the obtained ratio was multiplied by the concentration of the EPS in the culture sample taken. The pullulan content in the fermentation broth is expressed as g/L.
Determination of substrate concentration
After removing the pullulan by ethanol precipitation from the cell-free broth, the obtained solution was used for the estimation of residual sugar content [12]. DNS method was used for the determination of glucose, fructose, galactose, and lactose concentrations in fermentation media, whereas the phenol–sulfuric acid method was used for the determination of sucrose [14, 15].
Calculation of fermentation parameters
In this study, the maximum biomass, EPS and pullulan concentrations, specific microbial growth rates (μ), maximum pullulan formation rates (qp), substrate consumption rate (rs), and pullulan yield percentages were calculated in the fermentation experiments. The specific microbial growth rate for the exponential growth phase was calculated from the semi-logarithmic plot of the dry mass data versus time. The maximum pullulan formation rate was calculated according to Eq. (1);
where X is the dry biomass of A. pullulans AZ-6, Cp is the pullulan concentration, and t is time.
The substrate consumption rates (rs) were calculated by finding the slope values at different times from the curves in graphs showing substrate concentrations’ changes with time.
In experiments for optimization, pullulan yield (Y5, %) was calculated according to Eq. (2);
where Cpm is the highest pullulan concentration obtained during fermentation, SS0 is the initial amount of sucrose in the fermentation medium, and SS is the amount of sucrose at the time when the highest pullulan concentration is achieved.
All calculations were performed using Origin Pro 8.5 (Originlab Corporation, Northampton, USA) software.
Experimental design
In this study, RSM was used in order to determine the effects of various fermentation parameters on the pullulan production of A. pullulans AZ-6 and to optimize the pullulan production. Initial sucrose concentration (20–100 g/L), initial peptone concentration (0–12 g/L), initial pH (2.5–8.5), and temperature (24–32 °C) of the fermentation medium were selected as the fermentation parameters (independent variables) of pullulan production. For the optimization study, a trial version of the Design Expert® 10.0.2 (Inc. Minneapolis, USA) software was used and an experimental design was created by using the CCRD method.
The experimental design was conducted at five levels coded as − 2, − 1, 0, + 1, + 2, and consisted of 30 experimental points with 24 different combinations, with 6 repetitions at the center point. The coded values (xi) were calculated according to the following equation:
where x is the actual variable and xmax and xmin are the maximum and minimum values of the actual variable [16]. The independent variables and their levels are given in Table 3.
In this study, maximum EPS (Y1; g/L) and maximum pullulan concentrations (Y2; g/L), specific growth rates of the microorganism, µ (Y3; h−1), maximum specific pullulan formation rates, qp [Y4; g pullulan/(g mo.h)] and pullulan yield (Y5; %) were chosen as dependent variables.
Statistical analysis
In this study, quadratic model equations that show the effects of independent variables on dependent variables were derived by using multiple regression analysis using the trial version of Design Expert®10.0.2 (Inc. Minneapolis, USA) software.
Model equations of all responses were compatible with the following quadratic model equation:
where y is a response, Xi and Xj are independent variables, β0 is a constant coefficient, and βj, βjj and βij are linear, quadratic, and bivalent regression coefficients, respectively [17].
The significance of the derived quadratic model for each response was evaluated by analysis of variance (ANOVA) combined with the Fisher test by using the software. For this purpose, it was examined whether the model was important (p < 0.05), the lack of fit of the model was insignificant (p > 0.05), and the value of R2 was greater than 0.75 at 95% confidence level. At this stage, the individual, quadratic and the binary effects of the variables were determined and the linear (βj), quadratic (βjj), and binary interaction (βij) coefficients with p values less than 0.05 were evaluated as important at the 95% confidence level. Then, 3-dimensional (3D) graphics showing the binary effects of the independent variables on the dependent variables (the other two variables remained constant at the center point) were drawn by the software and these plots were examined.
Optimization and validation of optimum conditions
The optimum levels of the variables were obtained by numerical analysis using the software. Numerical optimization finds a point that maximizes the desirability function. For this purpose, the function of “desirability” that ranges from zero to one was used after determining the targets for both dependent and independent variables. Since one of the most important objectives of this study was the production of high concentrations of pullulan, the targets for maximum EPS concentration (Y1) and the maximum pullulan concentration (Y2) were both set as “maximum”. In the optimization study, no target was specified for microorganism specific growth rate (Y3) and pullulan yield (Y5), and the maximum specific pullulan formation rate (Y4) was allowed to be “in range”. The targets were set for all independent variables as “in range”.
As a result of numerical optimization, different solutions (conditions) proposed by the software were evaluated. The solution with the highest desirability value was selected as the optimum. A parallel experiment was conducted in these conditions. These experiments were performed in a shaking water bath at a constant shaking rate of 100 strokes/min in 300 mL flasks in 150 mL media. In the experiments, analytic assays were conducted according to Sect. “Analytic assays”. The suitability of the obtained responses in the optimum conditions was evaluated by determining whether these values were between the maximum and minimum predicted values at the 95% confidence level.
Characterizations of EPS samples
To characterize EPS samples including high concentration of pullulan produced under optimum conditions, a series of analyses were carried out. First, their molecular structure characterized with a FT-IR spectrophotometer (Shimadzu, FT-IR-8101, Japan) by using a method of Mishra and Vuppu [18] with slight modifications. For this analysis, the dried 2 mg EPS sample was homogenized in an agate mortar and mixed with 80 mg of KBr homogenously. This mixture was then pelleted using a hydraulic press (Shimadzu, Japan). The FT-IR absorbance spectra were obtained in the wavenumber region of 4000–400 cm−1 with 40 scans at a resolution of 4.0 cm−1. The molecular structures of EPS samples were also determined by using a SEM (Quanta FEG 450, FEI, Amsterdam, Netherlands). FT-IR spectroscopy and SEM analysis were also conducted for standard synthetic (Sigma Chemical Co., St. Louis, MO, USA) and commercial pullulan (Hayashibara Co., Ltd.) samples.
The molecular weights of EPS samples were determined by using the relation between molecular weight and intrinsic viscosity [19, 20]. For this purpose, dilutions (1–5 g/100 mL) of pullulan samples were prepared in distilled water. The Brookfield DV-II Pro (Brookfield, Middleboro, MA) model viscometer with LV-II (62) type spindle was used to measure the viscosities (η) of these solutions at a mixing speed of 100 rpm at 20 °C.
The relative viscosities (ηr) of EPS solutions were determined by using Eq. (5).
where η is the viscosity of the EPS solution at 20 °C and η0 is the viscosity of the water at the same temperature.
Specific viscosities (ηsp) of EPS solutions were calculated by using Eq. (6).
The specific viscosity varies depending on the concentration of the solution, and this change is expressed by the Huggins correlation (Eq. (7)):
where kʹ is a Huggins constant, [η] is an intrinsic viscosity, c is the concentration of the solution, and ηsp/c ratio is a viscosity number or reduced specific viscosity. Intrinsic viscosity [η] is the y-intercept for the linear best fit to ηsp/C as a function of C (g/dL).
Mark–Houwink equation refers to the changes in the intrinsic viscosities of polymer solutions with the molecular weights of the polymers and is given in Eq. (8).
where K and αM are the Mark–Houwink constants and Mw is the molecular weight of the polymer. It is stated that the values of Mark–Houwink constants vary depending on the temperature, type of polymer, and solvent.
K and αM values for the pullulan solution in the water at 20 °C are 2.58 × 10−2 and 0.646, respectively. In this study, molecular weights of EPS samples were calculated using Eq. (9).
Results and discussion
Effects of different carbon sources
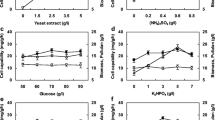
Variations in EPS, pullulan, biomass, and substrate concentrations and substrate consumption rate with time in the fermentation media including different carbon sources are represented in Fig. 2. When fructose was used as a substrate, EPS and pullulan concentrations maxima were obtained as 12.10 g/L at 144 h of fermentation (Fig. 2a, b). When glucose or galactose was used as a substrate, maximum EPS concentrations were determined as 11.1 and 5.8 g/L, respectively, at 168 h. The maximum EPS and pullulan concentrations were 11.8 and 11.0 g/L, respectively, in the fermentation medium containing sucrose as the carbon source. When glucose, galactose, or maltose was used as a substrate, maximum pullulan concentrations were 10.8 g/L, 5.2 g/L, and 3.2 g/L at 218, 168, and 144 h of fermentation, respectively.
The biomass concentrations (X) increased until 168 h of fermentation in media containing glucose, sucrose, or fructose, and then, the concentrations remained relatively constant (Fig. 2c). In fermentation media using fructose, glucose, and sucrose as substrates, the highest concentrations of biomass were 10.3, 9.4, and 9.3 g/L, respectively. In experiments using maltose or galactose as substrate, biomass concentration was found to increase rapidly during fermentation, and the highest biomass concentrations in these experiments were determined as 27.1 and 25.8 g/L at 218 h of fermentation, respectively.
In this study, specific growth rates (μ) of A. pullulans AZ-6 and maximum pullulan formation rates (qp) were also calculated. While specific growth rates of A. pullulans AZ-6 strain were 0.043, 0.047, 0.049, 0.023, and 0.017 h−1, specific product formation rates were 0.129, 0.122, 0.131, 0.048, and 0.060 g pullulan/(g mo.h) in fermentation media containing glucose, sucrose, fructose, maltose, and galactose, respectively. Figure 2d shows that glucose was consumed earlier (at the 168 h of fermentation) than other sugars, whereas maltose and galactose took longer time to be totally utilized (at the 218 h of fermentation). Changes in substrate consumption rates (rs) over time are presented in Fig. 2e. According to the results, the highest substrate consumption rate [1.16 g substrate/(L.h)] for A. pullulans AZ-6 was achieved when sucrose was used as the substrate in the fermentation medium.
In this study, among the tested substrates, sucrose, glucose, and fructose were found to support pullulan production comparatively superior to the others. In order to select the best substrate for pullulan production by A. pullulans AZ-6, these three substrates were tested again. In these experiments, the concentration maxima of EPS (15.8 g/L) and pullulan (15.7 g/L) were obtained in the fermentation medium containing sucrose as substrate (Fig. 3a, b). The maximum concentration of biomass (14.4 g/L) was obtained in the fermentation medium containing fructose (Fig. 3c). In these experiments, the specific growth rates (μ) of A. pullulans AZ-6 were also calculated as 0.032 h−1 for glucose and 0.037 h−1 for sucrose and fructose. Changes of substrate concentrations and substrate consumption rates (rs) with time for glucose, sucrose, or fructose are shown in Fig. 3d and e. In these runs, while glucose and fructose were consumed at 194 h of fermentation, sucrose was consumed at 216 h of fermentation. The sugar consumption rate (rs) maxima were calculated as 1.38, 0.60, and 0.75 g substrate/(L.h), respectively, for glucose, sucrose, and fructose.
The highest pullulan concentration in this study was obtained in the fermentation medium containing sucrose as a substrate. Therefore, sucrose was decided to be used as the carbon source in the next fermentation experiments. Similar to the results obtained from our study, it has been stated in the literature that the highest pullulan concentration is generally obtained when sucrose is used as a carbon source in the fermentation medium [21,22,23].
Effects of different nitrogen sources
The results of the experiments investigating the effects of different nitrogen source combinations used in the fermentation medium on the pullulan production of the A. pullulans AZ-6 strain are given in Fig. 4a–e.
When (NH4)2SO4 was a constant inorganic nitrogen source and peptone, urea, and yeast extract were tested as organic nitrogen sources, the highest EPS concentrations were 21.0, 16.4, and 16.3 g/L, respectively. The maximum EPS concentrations were 16.3 g/L for (NH4)2SO4, 15.2 g/L for NH4Cl, and 14.1 g/L for NaNO3 in experiments that evaluated the effects of various inorganic nitrogen sources on EPS production while maintaining yeast extract in the composition of the fermentation medium constant (Fig. 4a).
In experiments where the concentration of the inorganic nitrogen source in the composition of the fermentation medium was kept constant at 2.00 g (NH4)2SO4/L, it was determined that the highest pullulan was obtained in the medium containing peptone, followed by urea and yeast extract, respectively. The maximum pullulan concentrations in the fermentation media in which peptone and (NH4)2SO4 or yeast extract and NH4Cl were used together were obtained at 192 h of fermentation as 18.6 and 14.1 g/L, respectively (Fig. 4b). When yeast extract or urea was used as an organic nitrogen source, the highest pullulan concentrations were determined as 14.8 and 15.5 g/L at 170 h of fermentation, respectively. When the yeast extract and NaNO3 were used in combination, the concentration maxima of pullulan were obtained as 13.4 g/L at 240 h of fermentation.
Maximum dry biomass (X) was obtained as 13.0 g/L at the 240 h of fermentation in the experiment in which yeast extract and NaNO3 was used together, while the lowest biomass concentration (7.6 g/L) was obtained in the medium in which urea and (NH4)2SO4 was used as mixture. When (NH4)2SO4 or NH4Cl was used in the medium containing yeast extract as an organic nitrogen source, the highest biomass concentrations were determined as 12.1 and 11.2 g/L at the 240 h of fermentation, respectively (Fig. 4c).
In media containing yeast extract and (NH4)2SO4, peptone and (NH4)2SO4, or yeast extract and NH4Cl as a nitrogen source mixture, the maximum specific growth rate (μ) was calculated to be 0.039 h−1. As seen in Fig. 4d, in fermentation media where yeast extract and (NH4)2SO4 or yeast extract and NH4Cl were used in combination as nitrogen sources, sucrose was consumed completely by A. pullulans AZ-6 at the 192 h of fermentation, while urea and (NH4)2SO4, peptone and (NH4)2SO4 or yeast extract and NaNO3 were used as mixture, the substrate was completely consumed by the strain at the 240 h of fermentation. The highest specific product formation rates (qp) were 0.367 g pullulan/(g mo.h) in fermentation media containing yeast extract and (NH4)2SO4, peptone and (NH4)2SO4, or yeast extract and NH4Cl were used as nitrogen source mixtures. For fermentation media in which urea and (NH4)2SO4 or yeast extract and NaNO3 was used together, the highest specific product formation rates (qp) were calculated as 0.365 and 0.351 g pullulan/(g mo.h), respectively (Fig. 4e). In this study, since the highest pullulan concentration was achieved with the combination of peptone and (NH4)2SO4, these nitrogen sources were used in fermentation media in the next experiments.
Yoon et al. [24] conducted a study using Ashbya gossypii extract, meat extract, corn maceration liquor and powder, malt extract, soytone peptone, and yeast extract as an organic nitrogen source in fermentation media with the aim of optimizing pullulan production with A. pullulans IMS 822 strain. In this study, it was reported that the highest concentrations of pullulan and biomass were obtained in a fermentation medium containing A. gossypii extract. In another study, it was stated that ammonium sulfate, ammonium chloride, peptone, yeast extract, and malt extract were tested as nitrogen sources in the fermentation environment and the highest pullulan concentration was obtained when yeast extract was used [25].
Modeling of dependent variables by response surface methodology
A total of 30 experiments were carried out according to the experimental design created using the CCRD method. The results of these experiments were fitted with a second-order polynomial equation showing the effects of the initial sucrose concentration (X1), the initial peptone concentration (X2), the initial pH (X3), and the temperature (X4) on the maximum EPS concentration (Y1), the maximum pullulan concentration (Y2), the specific growth rate (Y3), the maximum specific pullulan formation rate of the A. pullulans AZ-6 (Y4), or the percentage of pullulan yield (Y5). All responses obtained from experiments in this context are given in Table 4.
When the data in Table 4 were analyzed, it was determined that the maximum EPS (Y1) and pullulan (Y2) concentrations ranged between 2.0 − 23.5 and 1.9 − 23.1 g/L, respectively, while the specific growth rate values of the A. pullulans AZ-6 (Y3), the maximum specific pullulan formation rates (Y4), and pullulan yields (Y5) ranged between 0.025–0.058 h−1, 0.021–0.059 [g pullulan/(g mo.h)], and 6.9–47.3%, respectively.
The second-order polynomial model equation showing the effects of independent variables on the maximum EPS concentration (Y1) is given in Eq. (10). In general, more than one-factor terms and higher-order terms in a model show interaction. The positive sign in front of the coefficients indicates the synergistic effect between the factor(s) and the dependent variable, and the negative sign indicates the antagonistic effect between the factor(s) and the dependent variable. In this case, while initial sucrose and peptone concentrations and the initial pH positively effect the maximum EPS concentration, temperature negatively effects it. The determination coefficient (R2) of the model equation (Eq. (10)) is 0.998. In general, a regression model having an R2 value higher than 0.9 is considered to have a very high correlation [7]. In this case, too, the R2 value is high enough to show that the actual values and the predicted values were pretty close (Fig. 5a).
The ANOVA table for the quadratic model of Y1 is shown in Table 5. The ANOVA results showed that the model was significant with an F-value of 529.13 and a lack of fit F-value of 2.97. The p-value of the model was 0.01. That meant the model was highly significant at a 95% confidence level. This means that the regression model provides an excellent explanation of the relationship between the independent variables and the response (maximum EPS concentration). In addition, it was determined that the linear effects of all independent variables on the maximum EPS concentration, the interaction of these parameters with each other, and their quadratic effects (p < 0.01) were also very important. It was noted that the lack of fit for this model was insignificant (p > 0.05) as desired.
The relationship between the independent variables and maximum EPS concentration (Y1) was further investigated using contour and 3D response surface plots (Fig. 6). Each graph represents the effects of two of the independent variables while the other two are held constant at the center value.
The contour and response surface plots of maximum EPS concentration (Y1) as the function of a initial sucrose (X1) and peptone concentrations (X2), b initial sucrose concentration (X1) and initial pH (X3), c initial sucrose concentration (X1) and temperature (X4), d initial peptone concentration (X2) and initial pH (X3), e initial peptone concentration (X2) and temperature (X4) and f initial pH (X3) and temperature (X4)
As can be seen from the values in Fig. 6a, the maximum EPS concentration in the fermentation medium decreased with increasing initial sucrose concentration in conditions where the initial peptone concentration was low. It is clear from Fig. 6a that the maximum EPS production (25.3 g/L) obtained when initial pH and temperature were their center value and initial sucrose and peptone concentrations were their highest level. In conditions where the initial pH and the peptone concentrations of the fermentation medium were 8.5 and 6 g/L, respectively, when the initial sucrose concentration increased, the maximum EPS concentration also increased considerably and reached the value maxima (Fig. 6b). Figure 5c shows that an increase in initial sucrose concentration at 24 °C caused an increase in EPS concentration. When the contour plot in Fig. 6d was examined, it was determined the maximum EPS concentration was lower than 10 g/L in operating conditions where the initial pH varied in the range of 2.5–4.3, the temperature was 28 °C, and the initial sucrose and peptone concentrations were 60 and 6 g/L, respectively. Figure 6e shows that the EPS concentration increased from 0.8 to 18.4 g/L when the temperature was decreased from 32 to 24 °C at the center value of sucrose and peptone concentrations. When the contour graphic in Fig. 6f was examined, it was determined that the maximum EPS concentrations obtained in operating conditions where the initial pH of the fermentation medium varies in the range of 7–8.5 and the temperature in the range of 24–27 °C are higher than 15 g/L.
The quadratic multiple regression model equation showing the effects of the factors on the maximum pullulan concentration (Y2) is shown in Eq. (11).
The determination coefficient for the model equation was R2 = 0.995. Figure 5b compares experimental data with the predicted values obtained from the statistical model in Eq. (2). It is clear that most points are close to the line adjustment, which means that the values determined experimentally are quite similar to those determined by the model. The ANOVA results of this model are shown in Table 6. This model was found quite significant (p < 0.01) at a 95% confidence level according to the variance analysis results. Besides, it was determined that the linear effects of all independent variables on the maximum pullulan concentration, the quadratic effects of these parameters, and their interactions with each other were also very important (p < 0.01). The lack of fit of the derived model was found to be insignificant (p > 0.05), which means a good indication of the model fit.
The contour and 3D response surface plots of the quadratic model are shown in Fig. 7. According to Fig. 7a, at 28 °C and initial pH 5.5, the concentration maxima of the pullulan were obtained when the concentrations of sucrose and peptone were high within the studied range. When the sucrose concentration in the fermentation medium was ranged from 20 to 100 g/L and peptone concentration was 6 g/L, it was observed that low pH negatively effected the pullulan concentration at 28 °C. According to Fig. 7b, the highest pullulan concentrations were obtained under conditions in which the initial pH of the medium ranged between 7 and 8.5 and the initial sucrose concentration ranged from 90 to 100 g/L. As seen in Fig. 7c, the maximum pullulan concentrations decreased by increasing the temperature from 24 to 32 °C when the initial sucrose concentration was 100 g/L. It was also determined that in conditions where the initial peptone concentration was between 0 and 10 g/L, the maximum pullulan concentration increased significantly with the increase in the initial pH value of the medium (Fig. 7d). The response surface and contour graphs showing the effects of initial peptone concentration of fermentation medium and the temperature on the maximum pullulan concentration are shown in Fig. 7e. As seen in Fig. 7e, pullulan concentration was quite low (under 5 g/L) at 32 °C when the initial peptone concentration of the media was between 7.5 and 12.0 g/L and the initial sucrose concentration and pH of the medium is 60 g/L and 5.5, respectively. It was also found that the maximum pullulan concentration was above 15 g/L when initial the pH was 8.5 and the temperature was between 24 and 26 °C (Fig. 6f).
The contour and response surface plots of pullulan concentration (Y2) as the function of a initial sucrose (X1) and peptone concentrations (X2), b initial sucrose concentration (X1) and initial pH (X3), c initial sucrose concentration (X1) and temperature (X4), d initial peptone concentration (X2) and initial pH (X3), e initial peptone concentration (X2) and temperature (X4) and f initial pH (X3) and temperature (X4)
The model equation showing the effects of the initial sucrose concentration (X1), the initial peptone concentration (X2) and the initial pH (X3) of the fermentation medium and the temperature (X4) on the specific growth rate (Y3) of the microorganism is presented in Eq. (12).
The R2 value for Eq. (12) that was obtained by using multiple regression methods was calculated as 0.987. The R2 value of the correlation equation very close to unity indicates that the experimental Y3 values and the predictive Y3 values calculated using this equation are very close to each other. Figure 5c demonstrates the good agreements between the predicted and actual the specific growth rate values. The variance analysis results of the derived model are given in Table 7. According to the variance analysis results, the model was found to be significant at the 95% confidence level (p < 0.01), while the lack of fit of the model was found to be insignificant (p > 0.05). It was determined that the linear effects of all independent variables on specific growth rate were significant (p < 0.05). It was also found that the interactive effects of the initial sucrose concentration and temperature and the initial peptone concentration and the initial pH and the quadratic effect of the initial peptone concentration on Y3 were insignificant (p > 0.05).
3D response surface and contour graphs showing the effects of initial sucrose and peptone concentrations, and initial pH of the fermentation medium, and temperature on the specific growth rate of the A. pullulans AZ-6 are presented in Fig. 8. The 3D surface and contour plots of the combined effect of initial sucrose and peptone concentrations of the medium on the specific growth rate at a constant pH of 5.5 and temperature of 28 °C are shown in Fig. 8a. When the results in the graph were examined, it was found that the microorganism specific growth rate reached higher values at 28 °C when initial pH was 5.5 and initial sucrose and peptone concentrations of fermentation media were higher than 60 and 7.5 g/L, respectively. In fermentation medium with an initial sucrose concentration of 100 g/L, the specific growth rate was higher than 0.06 h−1 when the initial pH of the fermentation medium varied within the range of 6–8.5 at 28 °C (Fig. 8b). It was observed that the specific growth rate of the microorganism increased with the increase in the temperature in the conditions where the initial pH and peptone concentration values of the fermentation medium were at the center point and the sucrose concentration ranged between 20 and 100 g/L (Fig. 8c). When the contour and response surface graphs in Fig. 8d were examined, it was found that when the initial peptone concentration in the fermentation medium changed between 0 and 12 g/L, the microorganism specific growth rates were increased by increasing the initial pH value of the medium at 28 °C. In this study, it was also found that the specific growth rate of the microorganism increased by increasing the initial concentration of peptone in the fermentation medium with an initial pH of 5.5 and a sucrose concentration of 60 g/L at approximately 24–27 °C (Fig. 8e). When the effect of initial pH, temperature, and their interaction on the specific growth rate of the microorganism at a fixed central level of initial sucrose and peptone concentrations was observed, it was found that the specific growth rate of the microorganism increased with the increase in the initial pH of the fermentation medium at 24 °C (Fig. 8f).
The contour and response surface plots of specific growth rate (Y3) as the function of a initial sucrose (X1) and peptone concentrations (X2), b initial sucrose concentration (X1) and initial pH (X3), c initial sucrose concentration (X1) and temperature (X4), d initial peptone concentration (X2) and initial pH (X3), e initial peptone concentration (X2) and temperature (X4) and f initial pH (X3) and temperature (X4)
The nonlinear multivariate regression equation showing the effects of the independent variables on the maximum specific pullulan production rate (Y4) is presented in Eq. (13).
The R2 value of this equation was calculated as 0.864 (Fig. 5d). The variance analysis results of the model equation are given in Table 8. According to the results of ANOVA, the model equation was found to be significant at a 95% confidence level (p < 0.05). The lack of fit of this model was found to be insignificant at a 95% confidence level (p > 0.05).
3D response surface and contour graphics showing the effects of independent variables on the maximum specific pullulan formation rate (Y4) are given in Fig. 9. The convex response surfaces in these graphs well define the values of the variables in which the maximum Y4 value is obtained and it is seen that the highest Y4 values are obtained at the center point of the independent variables. The maximum specific pullulan formation rate was found to be 0.055 [g pullulan/(g mo.h)] at the center point conditions (initial sucrose concentration; 60 g/L, initial peptone concentration: 6 g/L, initial pH: 5.5 and temperature: 28 °C).
The contour and response surface plots of maximum specific pullulan formation rate (Y4) as the function of a initial sucrose (X1) and peptone concentrations (X2), b initial sucrose concentration (X1) and initial pH (X3), c initial sucrose concentration (X1) and temperature (X4), d initial peptone concentration (X2) and initial pH (X3), e initial peptone concentration (X2) and temperature (X4) and f initial pH (X3) and temperature (X4)
The second-order nonlinear model equation showing the effects of initial sucrose concentration (X1), the initial peptone concentration (X2), the initial pH value (X3), and the temperature (X4) of the fermentation medium on pullulan yield (Y5) is shown in Eq. (14).
The coefficient of determination for the derived model equation is calculated as R2 = 0.995. Since the R2 value is close to unity, it shows that the model can be considered as a good fit and there is a good agreement between the experimental and predicted yield from this model (Fig. 5e). ANOVA results of the model are presented in Table 9. According to the results of variance analysis, the model was found to be very important at a 95% confidence level (p < 0.01). It was also determined that the linear and quadratic effects of all independent variables on pullulan yield were very important (p < 0.01). When the effects of binary interactions of independent variables on pullulan yield were examined, it was found that only binary effect of the initial sucrose concentration and initial pH was found to be insignificant (p > 0.05). It was also determined that the lack of fit for this model was insignificant (p > 0.05).
3D response surface and contour graphs showing the effects of the independent variables examined in this study on pullulan yield are presented in Fig. 10. In this study, it was found that the pullulan yield increased in fermentation media containing high initial sucrose concentration with increasing initial concentration of peptone (Fig. 10a). When peptone concentration of the media is 6 g/L, pullulan yield increases and reaches high values by increasing the initial pH for low initial sucrose concentration at 28 °C (Fig. 10b). When surface and contour plots in Fig. 10c were examined, it was found that pullulan yield in the fermentation medium with an initial sucrose concentration of 20 g/L and a temperature of 24–32 °C was above 40%. Pullulan yield was found to be increased at 28 °C with an increase in the initial pH in peptone-free fermentation media with an initial sucrose concentration of 60 g/L (Fig. 10d). In the fermentation media where the initial concentration of sucrose and peptone concentration was 60 and 12 g/L, respectively, and the initial pH of the medium was 2.5, pullulan yield was found to less than 10% at 26.3–32 °C (Fig. 10e). In conditions where the initial sucrose and peptone values were at their center points and the pH was between 2.5 and 8.5, it was found that the change in temperature did not significantly effect pullulan yield (Fig. 10f).
The contour and response surface plots of maximum specific pullulan yield (Y5) as the function of a initial sucrose (X1) and peptone concentrations (X2), b initial sucrose concentration (X1) and initial pH (X3), c initial sucrose concentration (X1) and temperature (X4), d initial peptone concentration (X2) and initial pH (X3), e initial peptone concentration (X2) and temperature (X4) and f initial pH (X3) and temperature (X4)
Optimization of pullulan production by A. pullulans AZ-6 strain
The optimum conditions were chosen among 100 solutions with desirability function changed in the range of 0.398–0.977, considering the value of the desirability function as close to 1. The conditions where the value of desirability function was 0.977, the initial sucrose and peptone concentrations and the pH of the fermentation medium were 100 g/L, 11.305 g/L, and 6.481, respectively, and the temperature was 24.2 °C were determined as optimum among the solutions computed by the software. In the optimum conditions, the maximum EPS and pullulan concentrations to be expected to obtain were 37.078 and 35.372 g/L, the specific growth rate was 0.062 h−1, the maximum specific pullulan formation rate and the pullulan yield were 0.021 [g pullulan/(g mo.h)] and 53.7%, respectively.
In a study by Choudhury et al. [26], optimization of the fermentation medium composition using the response surface method was performed to increase pullulan production of the A. pullulans RBF 4A3 strain. It was stated that the optimum experimental conditions determined in this study were 15.5%, 3.68%, and 1.75% for glucose, yeast extract, and peptone concentrations, respectively, and the pullulan concentration obtained under these conditions was 70.43 g/L In another study, it was stated that the effects of the initial substrate concentration (X1), aeration rate (X2), and incubation time (X3) of the fermentation medium on pullulan production in an aerated reactor with A. pullulans DSM-2404 were investigated by the response surface methodology. Optimum conditions in the study were determined as initial substrate concentration: 95.2 g/L, aeration rate: 1.93 vvm, and incubation time: 5.36 days. It has been reported that the pullulan concentration to be obtained under these conditions was predicted as 38.7 g/L. In this study, it was stated that the concentration of pullulan obtained in the experiment conducted to verify the optimum conditions was 39.2 g/L.
Validation of optimum conditions
In order to verify the optimized process parameters, fermentation experiments were conducted under the optimum conditions predicted by the software, and maximum EPS and pullulan concentrations, specific growth rate, maximum specific pullulan formation rate, and pullulan yield in these experiments were obtained as 36.95 and 35.47 g/L, 0.064 h−1, 0.036 [g pullulan/(g mo.sa)] and 54.480%, respectively. These obtained experimental values of the dependent variables in optimum conditions and predicted values that were calculated using model equations are presented in Table 10, and these results were evaluated by using post-analysis menu in the software. The minimum and maximum values of the dependent variables that should be obtained in optimum conditions at 95% confidence level are also given in Table 10. When the results in this table were examined, it was determined that the experimental values of the dependent variables were very close to their predicted values and these values are between the highest and lowest values calculated by the software. With these results, the optimum conditions were statistically verified at a 95% confidence level.
Characterizations of pullulan samples
The FT-IR spectra of various functional groups of EPS samples produced by A. pullulans AZ-6 strain, commercial and standard pullulan are given in Fig. 11. As can be seen in Fig. 11, the FT-IR patterns of samples were quite similar that confirmed the presence of the chemical structure of pullulan in the EPS sample produced by A. pullulans AZ-6. When the obtained results were examined, it was determined that wide peaks (3453.2–3397.1 cm−1) showing the presence of repeating units of the –OH groups in the sugars were obtained in all samples. It was observed that another strong absorption in the evaluated peaks was obtained in the C–H strain bonds in the wavenumber range 2928.3–2922.5 cm−1 (Fig. 11). In all the samples studied, the peaks of O–C–O stretching, C–O–H bend, C–O–C, and C–O O stretching were measured at wavenumbers ranging from 1653.0–1618.5, 1456.4–1358.0, 1159.5–1111.1, and 1024.3–981.9 cm−1, respectively. In pure and commercial pullulan samples and in EPS samples produced by A. pullulans AZ-6 strains, peaks showing the α-configuration and varying in the wavelength range of 754.3–848 cm−1 could also be measured (Fig. 11).
The second test for the characterization of pullulan produced under optimum conditions was the investigation of the molecular structure by using the SEM. Figure 12a shows an SEM image of the reference synthetic pullulan and it shows that synthetic pullulan exhibited an amorphous (irregular) shape pattern 3-D network structure and the presence of macro porosity. In the present study, the pullulan synthesized by A. pullulans AZ-6 displayed similar amorphous surface shape with tightly gathered pores (Fig. 12b). Pores of pullulan structure are shown with arrows in Fig. 12.
In this study, it was determined that the molecular weight of the EPS sample, which was obtained during the verification of the optimum conditions and contained more than 90% pullulan, was 1.05 × 107. It is stated in the literature that the molecular weight of pullulan can vary between 1.5 × 104 and 1.0 × 107 depending on the culture conditions.
Conclusion
Pullulan is a high-value-added polymer that has become increasingly common in industrial-scale production and has a wide range of uses. As is the situation with the other biotechnological products, pullulan’s cost is a significant factor despite the fact that it is used in multiple industries. Optimization is one of the crucial procedures that can increase pullulan production and decrease production costs. Another factor to be considered is pullulan’s contamination with melanin during the fermentation process. Removal of melanin from pullulan increases labor and expense. Utilizing a strain that does not synthesize melanin during the production of pullulan resolves this issue entirely. In this study, a color-variant strain (non-pigmented or melanin free) was used and the pullulan production conditions of this strain were optimized. Thus, a strain that may be important for industrial pullulan production and high concentration pullulan production conditions are presented. However, pilot-scale trials are also required for industrial-scale production.
References
Singh RS, Saini GK (2012) Biosynthesis of pullulan and its applications in food and pharmaceutical industry. In: Satyanarayana T, Johri BN, Prakash A (eds) Microorganisms in sustainable agriculture and biotechnology, 1st edn. Springer, New York, pp 509–555
Mitić Ž, Nikolić GS, Cakić M, Premović P, Ilić L (2009) FTIR spectroscopic characterization of Cu(II) coordination compounds with exopolysaccharide pullulan and its derivatives. J Mol Struct 924:264–273. https://doi.org/10.1016/j.molstruc.2009.01.019
Singh RS, Singh H, Saini GK (2009) Response surface optimization of the critical medium components for pullulan production by Aureobasidium pullulans FB-1. Appl Biochem Biotechnol 152:42–53. https://doi.org/10.1007/s12010-008-8180-9
Ganduri V, Mangamuri UK, Babu RS, Rao KRSS (2017) Response surface optimization of fermentation parameters for pullulan elaboration from jaggery by Aureobasidium pullulans MTCC 2195. J Chem Pharm Sci 10:457–461
Chen L, Tian Y, Zhang Z, Tong Q, Sun B (2017) Effect of pullulan on the digestible, crystalline and morphological characteristics of rice starch. Food Hydrocoll 63:383–390. https://doi.org/10.1016/j.foodhyd.2016.09.021
Lin Y, Zhang Z, Thibault J (2007) Aureobasidium pullulans batch cultivations based on a factorial design for improving the production and molecular weight of exopolysaccharides. Process Biochem 42:820–827. https://doi.org/10.1016/j.procbio.2007.01.013
Jiang L (2010) Optimization of fermentation conditions for pullulan production by Aureobasidium pullulans using response surface methodology. Carbohydr Polym 79:414–417. https://doi.org/10.1016/j.carbpol.2009.08.027
Singh RS, Kaur N (2018) Biochemical and molecular characterization of a new pullulan producer Rhodosporidium paludigenum PUPY-06. J Appl Biol Biotechnol 6:28–37. https://doi.org/10.7324/JABB.2018.60106
Haghighatpanah N, Omar-Aziz M, Gharaghani M, Khodaiyan F, Hosseini SS et al (2022) Effect of mung bean protein isolate/pullulan films containing marjoram (Origanum majorana L.) essential oil on chemical and microbial properties of minced beef meat. Int J Biol Macromol 201:318–329. https://doi.org/10.1016/j.ijbiomac.2022.01.023
Aghaie E, Pazouki M, Hosseini MR, Ranjbar M, Ghavipanjeh F (2009) Response surface methodology (RSM) analysis of organic acid production for Kaolin beneficiation by Aspergillus niger. Chem Eng J 2–3:245–251. https://doi.org/10.1016/j.cej.2008.07.008
Mujdeci G, Arévalo-Villena M, Ozbas ZY, Briones Pérez A (2018) Yeast identification during fermentation of Turkish Gemlik olives. J Food Sci 5:1321–1325. https://doi.org/10.1111/1750-3841.14124
Sheng L, Tang G, Su P, Zhang J, Xiao Q et al (2016) Understanding the influence of Tween 80 on pullulan fermentation by Aureobasidium pullulans CGMCC1234. Carbohydr Polym. https://doi.org/10.1016/j.carbpol.2015.10.058
Sharma N, Prasad GS, Choudhury AR (2013) Utilization of corn steep liquor for biosynthesis of pullulan, an important exopolysaccharide. Carbohydr Polym 1:95
Miller GL (1959) Use of Dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 3:426. https://doi.org/10.1021/ac60147a030
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 3:350. https://doi.org/10.1021/ac60111a017
Baş D, Boyacı İH (2007) Modeling and optimization I: usability of response surface methodology. J Food Eng 3:836–845. https://doi.org/10.1016/j.jfoodeng.2005.11.024
Carley KM, Kamneva NY, Reminga J (2004) Response surface methodology center for computational analysis of social and organizational systems technical report. http://128.2.205.20/publications/papers/CMU-ISR-04-136.pdf. Accessed 01 dec 2015
Mishra B, Vuppu S (2013) A study on downstream processing for the production of pullulan by Aureobasidium pullulans- SB-01 from the fermentation broth. Res J Recent Sci 2277:2507
Pişkin E (1987) Polimer Teknolojisine Giriş. İnkilap Kitabevi, İstanbul
Saçak M (2012) Polimer Kimyası. Gazi Kitabevi Tic. Ltd. Şti., Ankara
Seviour RJ, Schmid F, Campbell BS (2011) Fungal exopolysaccharides. In: Popa V (ed) Polysaccharides in medicinal and pharmaceutical applications, 1st edn. Smithers, London, pp 89–144
Liu NN, Chi Z, Wang QQ, Hong J, Liu GL et al (2017) Simultaneous production of both high molecular weight pullulan and oligosaccharides by Aureobasdium melanogenum P16 isolated from a mangrove ecosystem. Int J Biol Macromol 102:1016. https://doi.org/10.1016/j.ijbiomac.2017.04.057
Yang J, Zhang Y, Chen L, Xin X, Yang J et al (2018) Statistical optimization of medium for pullulan production by Aureobasidium pullulans NCPS2016 using fructose and soybean meal hydrolysates. Molecules 6:1334. https://doi.org/10.3390/molecules23061334
Yoon S, Hong E, Kim S, Lee P, Kim M et al (2012) Optimization of culture medium for enhanced production of exopolysaccharide from Aureobasidium pullulans. Bioprocess Biosyst Eng 35:167. https://doi.org/10.1007/s00449-011-0620-1
Sugumaran KR, Gowthami E, Swathi B, Elakkiya S, Srivastava SN et al (2013) Production of pullulan by Aureobasidium pullulans from Asian palm kernel: a novel substrate. Carbohydr Polym 1:697. https://doi.org/10.1016/j.carbpol.2012.09.062
Choudhury AR, Bhattacharyya MS, Prasad GS (2012) Application of response surface methodology to understand the interaction of media components during pullulan production by Aureobasidium pullulans RBF-4A3. Biocatal Agric Biotechnol. https://doi.org/10.1016/j.bcab.2012.02.003
Acknowledgements
The authors thank the Scientific Research Projects Coordination Unit of Hacettepe University, Ankara, Turkey, for providing financial support (Project no. FHD-2015-5305). The author, Gamze Nur Mujdeci, also thanks the Scientific and Technological Research Council of Turkey for a scholarship during her PhD studies.
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK). Funding provided by Türkiye Bilimsel ve Teknolojik Araştırma Kurumu.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mujdeci, G.N., Bozdemir, M.T. & Ozbas, Z.Y. Experimental modeling and optimization of pullulan production by Aureobasidium pullulans AZ-6. Polym. Bull. 81, 9139–9180 (2024). https://doi.org/10.1007/s00289-024-05146-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-024-05146-8