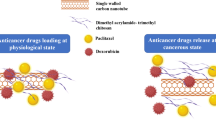
Abstract
The adsorption of the two anti-cancer drugs doxorubicin (Dox) and curcumin (Cur) and also the simultaneous loading of both drugs on the surface of the innovative inorganic nanostructure MXene/MOF-5 (Mxn-MOF) were investigated using the molecular dynamics (MD) simulation method. In order to study the loading process in presence of polymer, two chitosan and alginate polymers were adsorbed on the carrier, and then the loading of drugs was investigated. Descriptors such as van der Waals energy (vdW), radial distribution function (RDF), and mean square displacement (MSD) were utilized. The values of interaction energies and RDF for the studied systems show that the adsorption of drug molecules in systems containing polymer is better than in pristine ones. It was found that the type of adsorbed polymer on the Mxn-Mof nanostructure has a noticeable effect on the interaction energy between Cur and Dox drugs with the carrier. The obtained results confirmed that increasing the number of drug molecules affects the loading and adsorption process. The analyses show that in the co-loading system, the most stable complex with an average binding energy of − 662.75 kJ/mol belongs to the Mxn-MOF-Chi-Dox/Cur system. The investigation of the studied systems confirms that in the presence of chitosan polymer, the adsorption of drug molecules is stronger in comparison with alginate polymer. The results obtained from this study provide detailed information about the interaction of polymeric drug compounds and nanocarriers at the atomic level, which can be useful in the design of intelligent drug delivery systems.















Similar content being viewed by others
Data availability
Authors can confirm that all relevant data are included in the article and/or its supplementary information files.
References
Ratemi E (2018) pH-Responsive polymers for drug delivery applications. In: Stimuli responsive polym. nanocarriers drug deliv. appl., vol 1. Elsevier, pp 121–141
Cheng R, Feng F, Meng F, Deng C, Feijen J, Zhong Z (2011) Glutathione-responsive nano-vehicles as a promising platform for targeted intracellular drug and gene delivery. J Control Release 152:2–12
Hajebi S, Rabiee N, Bagherzadeh M, Ahmadi S, Rabiee M, Roghani-Mamaqani H, Tahriri M, Tayebi L, Hamblin MR (2019) Stimulus-responsive polymeric nanogels as smart drug delivery systems. Acta Biomater 92:1–18
Nik AB, Zare H, Razavi S, Mohammadi H, Ahmadi PT, Yazdani N, Bayandori M, Rabiee N, Mobarakeh JI (2020) Smart drug delivery: capping strategies for mesoporous silica nanoparticles. Microporous Mesoporous Mater 299:110115
Farokhzad OC, Langer R (2009) Impact of nanotechnology on drug delivery. ACS Nano 3:16–20
Debbage P (2009) Targeted drugs and nanomedicine: present and future. Curr Pharm Des 15:153–172
Kang Y, Zhang X-M, Zhang S, Ding L-S, Li B-J (2015) pH-responsive dendritic polyrotaxane drug-polymer conjugates forming nanoparticles as efficient drug delivery system for cancer therapy. Polym Chem 6:2098–2107
Naguib M, Kurtoglu M, Presser V, Lu J, Niu J, Heon M, Hultman L, Gogotsi Y, Barsoum MW (2011) Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv Mater 23:4248–4253
Ren CE, Hatzell KB, Alhabeb M, Ling Z, Mahmoud KA, Gogotsi Y (2015) Charge-and size-selective ion sieving through Ti3C2T × MXene membranes. J Phys Chem Lett 6:4026–4031
Seh ZW, Fredrickson KD, Anasori B, Kibsgaard J, Strickler AL, Lukatskaya MR, Gogotsi Y, Jaramillo TF, Vojvodic A (2016) Two-dimensional molybdenum carbide (MXene) as an efficient electrocatalyst for hydrogen evolution. ACS Energy Lett 1:589–594
Tang Q, Zhou Z, Shen P (2012) Are MXenes promising anode materials for Li ion batteries? Computational studies on electronic properties and Li storage capability of Ti3C2 and Ti3C2X2 (X = F, OH) monolayer. J Am Chem Soc 134:16909–16916
Wang H, Wu Y, Yuan X, Zeng G, Zhou J, Wang X, Chew JW (2018) Clay-inspired MXene-based electrochemical devices and photo-electrocatalyst: state-of-the-art progresses and challenges. Adv Mater 30:1704561
Huang K, Li Z, Lin J, Han G, Huang P (2018) Two-dimensional transition metal carbides and nitrides (MXenes) for biomedical applications. Chem Soc Rev 47:5109–5124
Lei J-C, Zhang X, Zhou Z (2015) Recent advances in MXene: preparation, properties, and applications. Front Phys 10:276–286
Rabiee N, Bagherzadeh M, Jouyandeh M, Zarrintaj P, Saeb MR, Mozafari M, Shokouhimehr M, Varma RS (2021) Natural polymers decorated MOF-MXene nanocarriers for co-delivery of doxorubicin/pCRISPR. ACS Appl Bio Mater 4:5106–5121
Rasool K, Mahmoud KA, Johnson DJ, Helal M, Berdiyorov GR, Gogotsi Y (2017) Efficient antibacterial membrane based on two-dimensional Ti3C2Tx (MXene) nanosheets. Sci Rep 7:1–11
Mayerberger EA, Street RM, McDaniel RM, Barsoum MW, Schauer CL (2018) Antibacterial properties of electrospun Ti3C2Tz (MXene)/chitosan nanofibers. RSC Adv 8:35386–35394
Lin H, Wang X, Yu L, Chen Y, Shi J (2017) Two-dimensional ultrathin MXene ceramic nanosheets for photothermal conversion. Nano Lett 17:384–391
Feng W, Wang R, Zhou Y, Ding L, Gao X, Zhou B, Hu P, Chen Y (2019) Ultrathin molybdenum carbide MXene with fast biodegradability for highly efficient theory-oriented photonic tumor hyperthermia. Adv Funct Mater 29:1901942
Rosi NL, Eckert J, Eddaoudi M, Vodak DT, Kim J, O’Keeffe M, Yaghi OM (2003) Hydrogen storage in microporous metal-organic frameworks. Science (80-) 300:1127–1129
Li H, Eddaoudi M, O’Keeffe M, Yaghi OM (1999) Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 402:276–279
Eddaoudi M, Kim J, Rosi N, Vodak D, Wachter J, O’Keeffe M, Yaghi OM (2002) Systematic design of pore size and functionality in isoreticular MOFs and their application in methane storage. Science (80-.) 295:469–472
Hasan MM, Hossain MM, Chowdhury HK (2021) Two-dimensional MXene-based flexible nanostructures for functional nanodevices: a review. J Mater Chem A 9:3231–3269
Martins CVB, Da Silva DL, Neres ATM, Magalhaes TFF, Watanabe GA, Modolo LV, Sabino AA, De Fátima A, De Resende MA (2009) Curcumin as a promising antifungal of clinical interest. J Antimicrob Chemother 63:337–339
Zorofchian Moghadamtousi S, Abdul Kadir H, Hassandarvish P, Tajik H, Abubakar S, Zandi K (2014) A review on antibacterial, antiviral, and antifungal activity of curcumin. Biomed Res Int 1–12
Lee W-H, Loo C-Y, Bebawy M, Luk F, Mason RS, Rohanizadeh R (2013) Curcumin and its derivatives: their application in neuropharmacology and neuroscience in the 21st century. Curr Neuropharmacol 11:338–378
Kunwar A, Barik A, Sandur SK, Indira Priyadarsini K (2011) Differential antioxidant/pro-oxidant activity of dimethoxycurcumin, a synthetic analogue of curcumin. Free Radic Res 45:959–965
Kunwar A, Jayakumar S, Srivastava AK, Priyadarsini KI (2012) Dimethoxycurcumin-induced cell death in human breast carcinoma MCF7 cells: evidence for pro-oxidant activity, mitochondrial dysfunction, and apoptosis. Arch Toxicol 86:603–614
Gupta SC, Patchva S, Aggarwal BB (2013) Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS J 15:195–218
Chandran B, Goel A (2012) A randomized, pilot study to assess the efficacy and safety of curcumin in patients with active rheumatoid arthritis. Phyther Res 26:1719–1725
Chen J, He Z-M, Wang F-L, Zhang Z-S, Liu X, Zhai D-D, Chen W-D (2016) Curcumin and its promise as an anticancer drug: an analysis of its anticancer and antifungal effects in cancer and associated complications from invasive fungal infections. Eur J Pharmacol 772:33–42
Nagy LI, Fehér LZ, Szebeni GJ, Gyuris M, Sipos P, Alföldi R, Ózsvári B, Hackler L, Balázs A, Batár P, Kanizsai I et al (2015) Curcumin and its analogue induce apoptosis in leukemia cells and have additive effects with bortezomib in cellular and xenograft models. Biomed Res Int 1–11
Sandur SK, Pandey MK, Sung B, Ahn KS, Murakami A, Sethi G, Limtrakul P, Badmaev V, Aggarwal BB (2007) Curcumin, demethoxycurcumin, bisdemethoxycurcumin, tetrahydrocurcumin and turmerones differentially regulate anti-inflammatory and anti-proliferative responses through a ROS-independent mechanism. Carcinogenesis 28:1765–1773
Hashemzadeh H, Raissi H (2020) Understanding loading, diffusion and releasing of Doxorubicin and Paclitaxel dual delivery in graphene and graphene oxide carriers as highly efficient drug delivery systems. Appl Surf Sci 500:144220
Karnati KR, Wang Y (2018) Understanding the co-loading and releasing of doxorubicin and paclitaxel using chitosan functionalized single-walled carbon nanotubes by molecular dynamics simulations. Phys Chem Chem Phys 20:9389–9400
Lázaro IA, Lázaro SA, Forgan RS (2018) Enhancing anticancer cytotoxicity through bimodal drug delivery from ultrasmall Zr MOF nanoparticles. Chem Commun 54:2792–2795
Wang J, Ma W, Tu P (2015) Synergistically improved anti-tumor efficacy by co-delivery doxorubicin and curcumin polymeric micelles. Macromol Biosci 15:1252–1261
Nazari SA, Farzad F, Haghi A, Bina A (2021) Surface functionalization of boron nitride nanosheet with folic acid: toward an enhancement in Doxorubicin anticancer drug loading performance. J Mol Graph Model 109:108041
Haghi A, Raissi H, Hashemzadeh H, Farzad F (2020) Designing a high-performance smart drug delivery system for the synergetic co-absorption of DOX and EGCG on ZIF-8. RSC Adv 10:44533–44544
Muthoosamy K, Abubakar IB, Bai RG, Loh H-S, Manickam S (2016) Exceedingly higher co-loading of curcumin and paclitaxel onto polymer-functionalized reduced graphene oxide for highly potent synergistic anticancer treatment. Sci Rep 6:1–14
Bina A, Raissi H, Hashemzadeh H, Farzad F (2021) Conjugation of a smart polymer to doxorubicin through a pH-responsive bond for targeted drug delivery and improving drug loading on graphene oxide. RSC Adv 11:18809–18817
Farzad F, Hashemzadeh H (2020) Probing the effect of polyethene glycol on the adsorption mechanisms of Gem on the hexagonal boron nitride as a highly efficient polymer-based drug delivery system: DFT, classical MD and Well-tempered Metadynamics simulations. J Mol Graph Model 98:107613
Lee J, Choi M-K, Song I-S (2023) Recent advances in doxorubicin formulation to enhance pharmacokinetics and tumor targeting. Pharmaceuticals 16:802
Lopez-Chavez E, Garcia-Quiroz A, Santiago-Jiménez JC, Díaz-Góngora JA, Díaz-López R, de Landa Castillo-Alvarado F (2021) Quantum--mechanical characterization of the doxorubicin molecule to improve its anticancer functions. MRS Adv 6:897–902
Kim S, Chen J, Cheng T, Gindulyte A, He J, He S, Li Q, Shoemaker BA, Thiessen PA, Yu B et al (2019) update: improved access to chemical data. Nucl Acids Res 47(2019):D1102–D1109
G.D. D’Ambruoso, M.E. Cremeens, B.R. Hendricks, Web-based animated tutorials using screen capturing software for molecular modeling and spectroscopic acquisition and processing, (2018).
Zoete V, Cuendet MA, Grosdidier A, Michielin O (2011) SwissParam: a fast force field generation tool for small organic molecules. J Comput Chem 32:2359–2368
Huang J, MacKerell AD Jr (2013) CHARMM36 all-atom additive protein force field: validation based on comparison to NMR data. J Comput Chem 34:2135–2145
Abraham MJ, Murtola T, Schulz R, Pá ll S, Smith JC, Hess B, et al (2015) GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1–2:19–25
Jorgensen WL, Chandrasekhar J, Madura JD, lmpey RW, Klein ML (1983) Comparison of simple potential functions for simulating liquid water. J Chem Phys 79:926–935
Bonati L, Zhang Y-Y, Parrinello M (2019) Neural networks-based variationally enhanced sampling. Proc Natl Acad Sci 116:17641–17647
Castaneda SMB, Alvarenga ES, Demuner AJ, Guimaraes LM (2020) Vibrational spectra and theoretical calculations of a natural pentacyclic triterpene alcool isolated from Mucuna pruriens. Struct Chem 31:599–607
Humphrey W, Dalke A, Schulten K et al (1996) VMD: visual molecular dynamics. J Mol Graph 14:33–38
William H (1996) VMD-visual molecular dynamics. J Mol Graph 14:33–38
Liu B, Goree J, Vaulina OS (2006) Test of the Stokes–Einstein relation in a two-dimensional Yukawa liquid. Phys Rev Lett 96:15005
Hibino H, Kageshima H, Kotsugi M, Maeda F, Guo F-Z, Watanabe Y (2009) Dependence of electronic properties of epitaxial few-layer graphene on the number of layers investigated by photoelectron emission microscopy. Phys Rev B 79:125437
Zaboli A, Raissi H, Farzad F, Hashemzadeh H (2020) Assessment of adsorption behavior of 5-fluorouracil and pyrazinamide on carbon nitride and folic acid-conjugated carbon nitride nanosheets for targeting drug delivery. J Mol Liq 301:112435
Torrik A, Zaerin S, Zarif M (2022) Doxorubicin and Imatinib co-drug delivery using non-covalently functionalized carbon nanotube: Molecular dynamics study. J Mol Liq 362:119789
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bina, A., Raissi, H. & Zaboli, A. Exploring the potential use of natural polymers to enhance the performance of MXene/MOF-5 nanocarrier in loading and co-loading of doxorubicin and curcumin. Polym. Bull. 81, 8383–8404 (2024). https://doi.org/10.1007/s00289-023-05099-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-023-05099-4