Abstract
The following paper presents a study of water dispersion obtained by emulsion co-polymerization of acrylic, methacrylic and vinyl monomers with the use of surfactants. Water dispersions of copolymers used in the production of paints intended for painting surfaces of steel metals and aluminum and its alloys were selected. They were synthesized by ethyl acrylate, butyl acrylate, acrylic acid, methyl methacrylate, methacrylic acid, acrylic acid amide, 2-hydroxyethylacrylate, glycidyl methacrylate, vinyl acetate and 1-ethyl-1-ethylpropylcarbamate (VeoVa 10). The investigated dispersion properties were considered in three groups. It turned out that the dispersions in which the copolymer had the lowest crystallinity temperature and were built of units having hydroxyl, carboxyl or amide groups were characterized by the most favorable properties. The hardness and weight of the membranes changed the least in the case of copolymers consisting of many units with functional groups.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Synthetic and natural polymeric dispersions are used in the production of paints, adhesives, membranes, foils, asphalt modifications, plaster mortars, textile and paper industries, etc. [1]. The use of emulsion polymerization represents an attractive path toward sustainable polymer production by replacing the hazardous VOCs used in solution polymerization [2,3,4,5]. Moreover, it is a versatile process which leads to the synthesis of polymers with unique properties [6, 7]. The emulsion polymerization process affects the scalability of the process, the division of radicals in nanoparticles leads to a high polymerization rate and polymers with a high molar mass [8,9,10]. Polymer additives using emulsion polymerization make it possible to improve the properties of cements, asphalts and plasters [11]. Mortars with better adhesion to the substrate, increased durability [12,13,14] and better elastic properties are obtained [15, 16]. The introduction of additional components to the polymer composition in the form of nanoparticles of materials increases the durability of water emulsions and their membranes [17, 18]. The properties of biodegradable poly(lactic acid) significantly increased after its modification with a dispersion of natural rubber [19]. Composites with optimal thermal properties, electrical conductivity and nanometric particle sizes were obtained as a result of emulsion copolymerization of polyaniline with natural rubber and acrylic monomers [20]. Additives in the form of zinc nano-oxide or carbon nanotubes introduced into polymer composites result in obtaining composites that conduct electricity well [21, 22]. Compositions of natural latex with additives are increasingly used in biomedicine due to their bactericidal properties [23,24,25]. Composites with good mechanical, thermal and magnetic properties were obtained from a dispersion of carboxylated styrene-butadiene rubber and carboxymethyl chitosan [26]. Dispersions showing higher stability during storage and processing were obtained as a result of emulsion copolymerization of ethylene with vinyl acetate [27].
The aim of the work was the synthesis of polymer dispersions by copolymerization of several monomers in an aqueous environment in the presence of one and the same surfactant. The tests consisted in determining the copolymerization conditions and the physicochemical properties of the dispersion. The research intention was to determine the influence of the composition of copolymers on the surface, coagulation and electrokinetic properties of polymeric dispersions and the physicomechanical properties of films obtained from them. An important challenge was to interpret the results of the research taking into account the properties of monomers, in particular the molar refractive index and crystallinity temperature of polymers and copolymers. Initial monomer compositions were determined on the basis of data contained in monographs [28,29,30]. They corresponded to polymer dispersions used for the production of primers, paints and enamels used for painting surfaces of steel metals as well as aluminum and its alloys. The monomers were ester, amide, hydroxyl, etc., derivatives of acrylic and methacrylic acid, and vinyl compounds. The tests consisted in determining the copolymerization conditions and the physicochemical properties of the dispersion. The research intention was to determine the influence of the composition of copolymers on the surface, coagulation and electrokinetic properties of polymeric dispersions and the physicomechanical properties of films obtained from them. An important challenge was to interpret the results of the research taking into account the properties of monomers, in particular the molar refraction and the crystallinity temperature of polymers and copolymers.
The literature review showed that the research issues presented in the paper had not been solved. In particular, it concerned the determination of the relationship between the electrokinetic and surface properties of particles in latexes and the physicomechanical properties of films formed from them and the structure of the copolymers themselves. The presented work is a continuation of research related to the demonstration of surface, coagulation, electrochemical and electrophoretic properties of latexes with practical application. Earlier, work was done on the study of the discussed properties of latices synthesized from ethyl and butyl acrylate, methyl and butyl methacrylate and methacrylic acid without surfactants [31].
Experimental part
Materials
Acrylic, methacrylic and vinyl monomers were used for latex syntheses. They were analytical grade products provided by Sigma-Aldrich. Table 1 presents their types and some properties.
The initiator of the free-radical emulsion co-polymerization was ammonium persulfate, an analytical grade reagent, a product of Avantor Performance Materials Poland in Gliwice. The surfactant was an aqueous solution containing 17.0 wt% of sodium kerylbenzene sulfonate (KBS). Iron (III) nitrate was an analytical grade reagent, a product of Avantor Performance Materials Poland in Gliwice. The monomers being used in the synthesis were distilled under reduced pressure.
Compositions of synthesized copolymers
Table 2 shows the compositions of the synthesized latexes. The applied amounts of monomers used are provided in mole parts.
Method of performing emulsion copolymerization
400 cm3 (22.22 g/mol) of water and the calculated amount of monomers were introduced into a four-neck round-bottom flask with a capacity of 1.0 dm3 equipped with a mechanical stirrer with adjustable speed, a reflux condenser and a thermometer. The flask was heated using an electric heating mantle. After the temperature reached 60 °C, the initiator (ammonium persulfate) has been added in small portions for three hours. The total amount of initiator was 1.2 g. The temperature was then increased to 70–75 °C and maintained for two hours until the end of the synthesis. After this time, steam distillation was performed to remove the remains of unreacted monomers from the latex. The latex was cooled down, filtered through a cotton septum, and used for testing. All latex syntheses were performed in the same manner [32].
Research methodology
Determination of co-polymerization yield and polymer concentration
5.0 cm3 of latex was measured into a Petri dish with a diameter of 10 cm weighed on an analytical balance and placed in a dryer with forced air circulation at 85.0 °C. Drying was continued until the water was completely removed. After drying a polymeric film was obtained. The Petri dish was cooled down and re-weighed. The weight of the resulting polymer was calculated based on the difference in weights. The co-polymerization yield was defined by the ratio of the weight of the polymer to the weight of monomers based on its amount in 5.0 cm3 of the latex sample. In turn, the ratio of the amount of polymer in the sample to the amount of the applied latex was its concentration [33].
pH measurement
The measurement consisted in immersing a glass combination electrode into a measuring cup with latex and reading out the pH value directly from the scale of the device. A CPC-505 pH meter with a combination IJ 44A electrode manufactured by Elmetron (Poland) was used for the tests [33].
Measurement of surface tension
The measurement of surface tension of latexes was performed using a K6 tensiometer provided by Krüss (Germany). The measurement consisted in determining the value of force needed to detach the platinum ring from the latex surface. The value of surface tension was read out directly from the scale of the instrument in mN/m. The instrument was checked prior to measuring the surface tension of the latexes. Several measurements of the surface tension of standard liquids were performed [33].
Viscosity testing
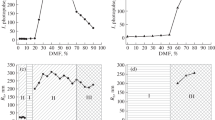
The viscosity of the latexes was measured with an Ubbelohde capillary viscometer. The measurement consisted in determining the time of the outflow of a specific volume of latex from the reservoir through the capillary. The intrinsic viscosity (IV) was determined based on the graphical dependency of the reduced viscosity on the polymer concentration in the latex. Based on the calculations including the Huggins equation, the parameter determining the interaction of water with the polymer, called Huggins constant (kH) [34], was determined. Figure 1 shows an example of the discussed dependency.
Determination of molecular masses of copolymers
Determination of molecular masses and polydispersity of the obtained copolymers were made by gel permeation chromatography. A Merck-Hitachi apparatus, consisting of an L-6200 pump, an L-5025 column thermostat, a D-2520 GPC integrator and a Merck RI-71 refractometric detector, was used. Preparing the test sample consisted in complete evaporation of water under reduced pressure at 60 °C and dissolution of the copolymer film in dimethylformamide. The standard was a solution of methyl acrylate copolymer with butyl acrylate and methacrylic acid (65:32:3% w/w) in dimethylformamide with a molecular mass of 2.03 × 105 [35].
Measurement of particle size in aqueous dispersions of copolymers
The particle size in the dispersion was determined with a Malvern Panalytical Mastersizer 3000 analyzer working based on laser diffraction using a HELOS measurement system. The test consisted in measuring the intensity of the scattered light after the laser beam passed through a sample containing dispersed polymer particles. Measurements by laser diffraction were performed in accordance with ISO 13320:2020.
Determination of coagulation thresholds
Coagulation tests of latexes were performed by conductometric titration. An iron (III) nitrate solution with a concentration of 0.0325 g/cm3 was used. Based on the curve of the dependence of the added amount of iron (III) nitrate solution on the conductivity, the values of two polymer coagulation thresholds in the latex were determined. The first threshold was non-overt coagulation (cp1) and indicated the association processes of polymer particles in the latex. The second threshold was overt coagulation (cp2), which represented the formation of flocculent structures created by associates of polymer particles. The flocculent structures of the combined polymer particles developed evenly throughout the volume of the latex. The unit of the coagulation threshold is the expression describing the amount of iron (III) ions per amount of polymer in the latex sample, or gFe(III)/g of polymer [36]. Figure 2 shows an exemplary relationship for a dispersion of composition 1.
Conductometric testing
The specific conductivity of the dispersion was measured with the OK-102/1 conductometer provided by Redelik’s (Hungary). The measurement consisted in immersing the electrode into the measuring cup with latex and reading out the conductivity value directly from the scale of the instrument. A bell electrode consisting of three platinum rings covered with platinum black was used for the measurement. The dependence of the equivalent conductivity (λr, mScm2/g) on the dispersion concentration was a straight line. The discussed relationship was described using the Kohlrausch–Onsager equation in the form [33, 35]:
The Kohlrausch–Onsager equation allowed for the determination of the limit value of the equivalent conductivity (λo) when c → 0 and the constant A. All calculations were performed in accordance with the methodology provided in [37]. An example of a relationship is shown in Fig. 3.
Electrophoretic testing
The process of electrophoretic deposition of polymer particles consisted in immersing two electrodes (anode and cathode) made of acid-resistant steel into the latex and switching on the direct current flow. Previously, the anode was weighed on an analytical balance. The voltage between the electrodes was 40.0 V. After five minutes, the final current value was recorded and the circuit was disconnected. The electrodes were removed from the latex. The amount of polymer deposited on the anode surface was determined by gravimetric analysis after it was dried at 75–80 °C to a constant weight and re-weighed on an analytical balance. Knowing the electrode surface covered with the polymeric film, the mass of polymer per 1 cm2 of the electrode surface was calculated. It was the so-called unit mass of the film on the electrode. The equivalent weight of the polymer released from the latex was calculated from the first Faraday’s law in the form [36, 37]:
where “m” is the mass of the film formed on the anode surface in mg, “i” is the current intensity in mA, “t” is the electrodeposition time in s, and “k” is the equivalent weight of the film-forming polymer deposited on the anode.
Using the Hittorf method, the potential of the polymer particles in the dispersion was calculated according to the following formula:
where “ζ” is the electrokinetic potential of the polymer particles in latex in V, “η” is latex viscosity in Ns/m2 (measured with a Höppler viscometer), “λ” is latex conductivity in µS/cm, “ε” is dielectric constant of the latex dispersion medium, “c” is polymer concentration in the latex in g/cm3, H = E/l where “E” is the voltage between the electrodes, and “l” is the distance between the electrodes (3 cm)
Determination of the relative hardness of the polymeric film
The determination of the relative hardness of the films produced from dispersion was performed with the use of the AWS-5 pendulum apparatus provided by Dozafil in Wrocław (Poland). The measurement of relative hardness consisted in the automatic counting of the number of deflections of the Persoz pendulum placed on the tested film to the number of deflections of the pendulum placed on a glass plate in a strictly defined range of its deflection angle. Polymeric films for hardness measurements were obtained by pouring dispersion onto glass plates with dimensions of 30 × 70 mm, previously weighed on an analytical balance and placed in template frames. The latex was dried at a temperature of 120° C for about 150 min to a constant weight of the film in a dryer with forced air circulation. The thickness of the films was between 150 and 200 µm. The tests were carried out in accordance with the PN-ISO 1522:2008 and PN-79/C-81530 standards and the data provided in [38].
Determination of resistance of polymeric films to hot water
The determination of the resistance of polymeric films to hot water consisted in preparing samples of films on glass plates according to the method described in Sect. ”Determination of the relative hardness of the polymeric film”. The glass plates with membranes were removed from the templates and re-weighed on an analytical balance. Subsequently, each sample was wrapped in tissue paper and immersed in distilled water at 60 °C. After 120 min, the samples were removed and dried to constant weight at 90–100 °C in a dryer with forced air circulation. The dried samples were re-weighed on an analytical balance. The change in mass of the membranes was calculated. Film mass loss was expressed as a percentage. The tests were carried out in accordance with the PN-EN ISO 2812-2-2:2019-01 standard and the data provided in monographs [34, 40].
Calculation of the molar refractive index of monomers, the Hildebrand solubility parameter and the crystallinity temperature of polymers and copolymers
The values of the Hildebrand solubility parameter of polymers and copolymers were calculated on the basis of data included in monographs [38,39,40]. The values of the crystallinity temperature of polymers obtained from the tested monomers were calculated and taken from the monograph [41]. The values of the crystallinity temperature of the copolymers were calculated from the formula.
where m1, m2, m3 and mn are the molar amounts of the monomer used in the synthesis of the copolymer (Table 2), Tk1, Tk2, Tk3 and Tkn are the crystallinity temperatures of the individual polymers (Table 1). The molar refractive values of the monomers were calculated on the basis of the Lorentz–Lorenz equation presented in monograph [41].
Results
The basic properties of the tested aqueous copolymer dispersions are presented in Table 3.
The presented properties of the aqueous dispersions were considered in three groups. The first group included dispersions from 1 to 4. The synthesized copolymers included vinyl acetate, acrylic acid, glycidyl methacrylate, acrylic acid amide, VeoVa, butyl and ethyl acrylate and 2-hydroxyethyl acrylate. These monomers were characterized by a high molar refractive index and the lowest crystallinity temperature of the copolymers (Table 3). The second group included dispersions from 5 to 8. The copolymers consisted of acrylic and methacrylic acid, ethyl and butyl acrylate, methyl methacrylate and 2-hydroxyethyl acrylate. The consequence of the presence of carboxyl and hydroxyl groups as well as ester substituents in the copolymers was a significant increase in their crystallinity temperature (Table 3). The third group included dispersions from 9 to 12. Dispersion no. 9 consisted of vinyl acetate, acrylic acid and acrylic acid amide showing a low molar refractive value and butyl acrylate, VeoVa and 2-hydroxyethyl acrylate with a very high molar refractive value. The consequence of such a composition of the copolymer was the lowest value of its crystallinity temperature (Table 3). The other three dispersions were made of butyl acrylate having a high molar refractive value and methacrylic acid and acrylic acid amide having a low molar refractive value. Studies of these dispersions have shown that the amount of methacrylic acid and acrylic acid amide in the copolymer significantly affects its crystallinity temperature. The values of the crystallinity temperature of these dispersions are higher than dispersion no. 3, and lower than dispersion no. 4 (Table 3). In the case of dispersion systems belonging to the first group, the highest values of copolymerization efficiency and polymer concentration were found in the second and third dispersions. These systems also had similar pH values. On the other hand, the highest value of surface tension had the first dispersion and the lowest—the third and fourth dispersion. Most likely, the increase in copolymerization efficiency in this group of latexes was influenced by the presence of the VeoVa monomer and 2-hydroxyethyl acrylate with the highest molar refractive index (Table 1). In particular, the hydroxyl group in the monomer may increase the efficiency of copolymerization and decrease the surface tension of the dispersion. In the case of dispersions in the second group, the highest value of copolymerization efficiency and polymer concentration corresponded to the eighth system. The copolymer in this dispersion had the highest crystallinity temperature (Table 3). Undoubtedly, it was related to the presence of carboxyl and hydroxyl groups in the monomers. After the copolymerization of the monomers used for the synthesis of the seventh dispersion, the lowest value of polymer yield and concentration was found, as well as the second highest crystallinity temperature of the copolymer. There was no 2-hydroxyethylacrylate in the composition of this copolymer. In the third group, there were dispersions in which the lowest value of copolymerization efficiency and relatively high pH had the tenth system, and the highest copolymerization efficiency had the ninth dispersion composed of vinyl acetate. The higher efficiency of copolymerization of the eleventh latex as compared to the twelfth latex is related to the fact that more methacrylic acid was used in the synthesis of the eleventh copolymer. The performed tests show that the copolymerization efficiency and the polymer content in the dispersion were strongly affected by the presence in the copolymer macromolecule of monomers derived from vinyl acetate, acrylic or methacrylic acid, 2-hydroxyethyl acrylate and acrylic acid amide showing hydrophilic properties. Figure 4 shows the dependence of intrinsic viscosity on particle size for individual dispersions.
Relationship between the viscosity number and the particle dimensions for individual (compositions is given in Table 2)
The data presented in Fig. 4 shows that the highest intrinsic viscosity values were when the copolymer consisted of glycidyl methacrylate, VeoVa and 2-hydroxyethylacrylate. These were generally the monomers with the highest molar refractive index and were part of the copolymers present in the second and third dispersions. The crystallinity temperatures of these copolymers were in the range of 160–200 K (Table 3). On the other hand, measurements of the size of particles formed from these copolymers also confirmed their largest sizes. In the second group, the highest value of intrinsic viscosity had the dispersion in which the copolymer contained acrylic acid and acrylic amide, and the lowest value had the copolymer with 2-hydroxyethylacrylate. The higher value of the intrinsic viscosity of the fifth copolymer dispersion compared to the other dispersions in this group can be explained by the similar molar refractive index of both monomers and very low crystallinity temperatures of the polymers formed from them. In the third group there were dispersions marked as tenth, eleventh and twelfth. They had the same qualitative composition but increasing amounts of acrylic acid amide and methacrylic acid. In this case, the smallest polymer particles were found to be present in the eleventh dispersion in which the copolymer had the lowest crystallinity temperature. Particle size measurements in dispersions by laser diffraction showed that dispersions in which intrinsic viscosities differ slightly contain particles of significantly different sizes. This is due to the fact that dispersion viscosity measurements are carried out under conditions of dynamic flow, i.e., the movement of liquid and polymer particles. On the other hand, particle size measurements by laser diffraction were carried out in static conditions in which their temporary association was possible. This meant that the formed associations of polymeric particles had a very unstable structure and were easily destroyed. Table 4 presents the results of molecular weight and polydispersity tests of synthesized copolymers present in dispersions.
The data presented in Table 4 shows that as the amount of monomers included in the copolymer increases, their molecular weight and polydispersity increase. This means that the polymer particles have larger dimensions and can form agglomerates consisting of smaller particles, as well as copolymers of different composition and structure. In such a situation, the structure of copolymer particles consisting of different units is heterogeneous and complex. In the first group of dispersions marked with numbers from 1 to 4, dispersion no. 1 was distinguished, obtained from vinyl acetate, glycidyl methacrylate and acrylic acid. It was characterized by the lowest molecular weight and polydispersity. It can be concluded that in this dispersion the polymer particles are the most homogeneous in terms of chemical composition, molecular weight and dimensions. For the synthesis of dispersions no. 2, 3 and 4, more monomers were used, increasing the starting compositions by acrylic amide, 2-hydroxyethylacrylate, VeoVa, butyl or ethyl acrylate. In this way, a dispersion was obtained in which the particles of the resulting polymer had the highest molecular weight and polydispersity. This proved that they were heterogeneous and most likely composed of agglomerates of smaller particles as well as polymeric particles composed of various monomers. The group of dispersions marked with numbers from 5 to 8 included such copolymers for the synthesis of which, among others, acrylic amide, acrylic acid, methacrylic acid and 2-hydroxyethylacrylate were used. The lowest molecular weight and the lowest polydispersity were found in those copolymers containing methacrylic acid and 2-hydroxyethylacrylic acid mers. In this case, it could be due to their significant hydrophilicity as well as very large differences in their molar refractive values. In the group of dispersions marked with numbers from 9 to 12, dispersion no. 9 deserves attention. Acrylic acid amide, acrylic acid and 2-hydroxyethylacrylate were used for its synthesis. The copolymer in this dispersion had a molecular weight and polydispersity as well as properties similar to latex No. 3 and 4. The copolymers in dispersions 10, 11 and 12 consisted of acrylic acid amide, butyl acrylate and methacrylic acid units. There were more acrylic acid amide units in the copolymer present in dispersion no. 10. On the other hand, in the copolymer present in dispersion no. 12, there were more methacrylic acid units. All copolymers were synthesized with the same amount of butyl acrylate. The molecular weight and polydispersity of the copolymer in dispersion no. 10 was lower than the molecular weight and polydispersity of the copolymer in dispersion no. 12. It was found that the molecular weight and polydispersity of the copolymer was influenced by the presence of acrylic acid amide units rather than methacrylic acid units. Figure 5 shows the dependence of the constant A from the Kohlrausch–Onsager equation on the constant kH from the Huggins equation for the tested aqueous copolymer dispersions.
Dependence of the constant A from the Kohlrausch–Onsager equation on the constant kh from the Huggins equation for the tested aqueous copolymer dispersions (compositions in Table 2)
Constant A from the Kohlrausch–Onsager equation characterizes the shape and structure of the polymer molecule. The most common particle shape of the dispersion was a sphere, but it can also be an irregular shape formed by particle associations or branches. In turn, the Huggins constant determined the interaction of the polymer with the aqueous environment. The tests showed that it showed the highest value in the case of the third dispersion when it contained the largest amount of 2-hydroxyethyl acrylate. The hydroxyl groups present in the polymer caused the greatest interactions between the surface of the copolymer particles and the aqueous environment. It can be concluded that the shape and different structure of the polymer particles was similar in the remaining dispersions. Therefore, no particular correlation was found between the discussed values. Figure 6 shows the dependence of the boundary value of the equivalent conductivity of water dispersions of copolymers on the anodic electrokinetic potential of their particles.
Dependence of the conductivity limit value of equivalent dispersion and the anode electrokinetic potential of copolymer particles (compositions is given in Table 2)
The data presented in Fig. 6 results in a certain regularity, consisting in the fact that the higher limit value of the dispersion equivalent conductivity corresponded to the higher value of the anodic electrokinetic potential of the polymer particles. It turned out that the highest values of the limiting equivalent conductance and anodic electrokinetic potential have particles of the copolymer which included 2-hydroxyethyl acrylate. It could be concluded that the almost constant limit value of the equivalent conductance corresponded to slightly increasing values of the anode electrokinetic potential. These were dispersions in which the copolymer particles contained methacrylic acid mer. In these cases, the potential of the polymer particle in the dispersion was determined by the degree of dissociation of carboxyl groups. The lowest values of the limiting equivalent conductivity and anodic electrokinetic potential showed the particles of the copolymer for the synthesis of which vinyl acetate and glycidyl methacrylate were used. One of the shortcomings of the measurements of the electrokinetic potential of particles using the electrophoretic method was the phenomenon of association of polymer particles caused by the flow of electric current through the dispersion. As a rule, larger polymer particles had a lower potential. In addition, amide and carboxyl groups were present in the chain of the polymer macromolecule. The surfactant used was of the anionic type and did not adsorb on the surface of the particle in places where carboxyl groups were present. Then, the electrokinetic potential of the polymer particle was lower. Figure 7 shows the dependence of the latent and overt coagulation values of the polymer particles on the type of dispersion.
Values of cover and overt coagulation of polymer particles depending on the type of dispersion (compositions are given in Table 2)
The data presented in Fig. 7 shows that in the first dispersion group, the highest values of the latent and overt coagulation threshold showed copolymers containing 2-hydroxyethylacrylate, acrylic amide and VeoVa. Also dispersions containing copolymers with the mentioned monomers had the highest values of limiting equivalent conductivity, the constant A from the Kohlrausch–Onsager equation and the anodic electrokinetic potential. It can be concluded that for these dispersions, the amide and hydroxyl groups present in the copolymer, as well as the presence of VeoVa, which can give the polymer particle a branched structure, played an extremely important role. It is possible that in this way the polymer particle in the dispersion additionally obtained steric stability. The second group of dispersions includes copolymers containing 2-hydroxyethylacrylate and methacrylic acid. These were monomers with a significant molar refractive index and a low crystallinity temperature of the forming polymers. In this group of dispersions, the values of the latent and overt coagulation thresholds as well as the boundary equivalent conductance and constant A from the Kohlrausch–Onsager equation are very similar to the dispersion of the first group. On the other hand, the values of the anode electrokinetic potential were slightly lower. The properties of dispersions included in the third group basically depended on the presence of mers derived from acrylic amide or methacrylic acid in the copolymer. In this case, the highest values of coagulation thresholds, limiting equivalent conductivity and electrokinetic potential were obtained. The research showed that the presence of units derived from ethyl acrylate, butyl acrylate or methyl methacrylate in the copolymer had minimal or almost no effect on the coagulation threshold values. Table 4 presents the results of testing the properties of the films obtained from the dispersion after evaporation of water from them and complete drying.
From the data presented in Table 4 it can be seen that the hardness of the films was very different. Before water extraction, the highest hardness values were found in the films from dispersion no. 1, 4, 6 and 9. The lowest values, however, corresponded to the films from dispersion no. 3, 5, 8, 10 and 12. In turn, after water extraction, the hardness of the films from dispersion no. 1, 4, 6, 8 and 9 increased the most. The films with dispersion no. 3, 5, 7, 10 and 12 had the lowest hardness. The tests showed that the hardness of those films in which the macromolecules contained mers with carboxyl, hydroxyl or amide groups changed the least. This proved that these membranes were the most resistant to water. Undoubtedly, during the water extraction, the surfactant was washed out of the film first. The analysis of the value of the Hildebrand parameter of copolymers in dispersions showed that the highest value had copolymers consisting of acrylic acid, acrylic amide, butyl acrylate and methyl methacrylate. These were dispersions no. 1, 2, 3, 5 and 8. The lowest values of the Hildebrand parameter were found in copolymers containing in their composition mers with functional groups or substituents derived from methacrylic acid and acrylic amide. These included, among others, dispersions no. 4 and 11. Films made of copolymers containing the mentioned monomers showed the smallest changes in hardness and weight.
Discussion
The properties of the copolymers in the first dispersion group depended on the presence of VeoVa and 2-hydroxyethyl acrylate units in their macromolecule. The second group included copolymers whose properties were affected by the presence of units derived from acrylic acid and acrylic amide. The third group consisted of copolymers whose properties depended on the units derived from vinyl acetate, acrylic acid, VeoVa, acrylic amide and 2-hydroxyethylacrylate. The efficiency of copolymerization and the polymer concentration in the dispersion were strongly influenced by vinyl acetate, acrylic or methacrylic acid, 2-hydroxyethylacrylate and acrylic acid amide. The tests confirmed that the largest particles were present in the dispersions in which the copolymers consisted of units forming polymers with low crystallinity temperatures. As the amount of monomers included in the copolymer increased, their molecular weight and polydispersity increased. The lowest molecular weight and polydispersity, i.e., the highest homogeneity, had the copolymer obtained from vinyl acetate, glycidyl methacrylate and acrylic acid. In turn, the highest molecular weight and polydispersity were found in the copolymers obtained with acrylic amide, 2-hydroxyethyl acrylate, VeoVa, butyl or ethyl acrylate. One of the reasons could be their monomer molecular weight and different polymerization reaction energy. The values of the anode electrokinetic potential and the boundary equivalent conductivity depended on the structure of the copolymer. They had the highest values in dispersions in which 2-hydroxyethylacrylate, acrylic or methacrylic acid were used for the synthesis of copolymers. Undoubtedly, their stability, and in particular coagulation, was affected by the value of the potential and conductivity of the polymer particles as well as the presence of a surfactant. The tests of hardness of polymer films and changes in their weight after water extraction confirmed the essential role of functional groups in copolymers. The presence of a hydroxyl, amide or carboxyl group in the copolymer favored the formation of polymeric films more resistant to water.
Conclusion
The research showed that in dispersions in which copolymers consisted of units forming polymers with low crystallinity temperatures, the largest particles were present. Increasing the number of units in the copolymer increased its molecular weight and polydispersity. In the dispersions where the copolymers had the lowest molecular weight, the polymer particles were the most homogeneous. In turn, the highest polydispersity was found in copolymers obtained from many monomers significantly differing in molar refractive index and crystallinity temperature. The values of the anodic electrokinetic potential, limiting equivalent conductance and coagulation stability depended on the presence of hydroxyl and carboxyl groups in the copolymer as well as the presence of the surfactant. The hardness of the membranes and changes in their weight after water extraction depended on the presence of functional groups in the copolymer that favor the formation of water-resistant membranes. However, in reference to the previous work [33], we can conclude that the best latex without surfactants was a copolymer consisting of ethyl acrylate, methyl methacrylate and methacrylic acid. Among the tested latices in which the copolymers consisted of acrylic acid amide, 2-hydroxyethyl acrylate, butyl or ethyl acrylate and methacrylic acid with the addition of a surfactant, we can also conclude that their electrokinetic properties are similar to the copolymer synthesized without the participation of a surfactant. Their properties predispose for potential use in paints for electrophoretic painting.
Data availability
The raw/processed data required to reproduce these findings cannot be shared at this time due to technical or time limitations.
References
Blackley DC (1977) Polymer latices, Vol. 1–3, Springer Netherlands, Amsterdam
Zhang K, Li L, Chen X, Lu C, Ran J (2022) Controlled preparation and properties of acrylic acid epoxy‐acrylate composite emulsion for self‐crosslinking coatings. J Appl Polym Sci 139(1):51441
Qiu Z, Wang L, Wu J, Li W, Gu T (2021) Study the synthesis and the properties of self‐crosslinking acrylic latex via a novel fluorescent labeling method. J Appl Polym Sci 138(10):49973
Gonzalez-Martin J, Contera S, Lebrero R, Munoz R (2022) Optimization of acrylic-styrene latex-based biofilms as a platform for biological indoor air treatment. Chemosphere 287:132182
Zhang C, Luo J, Yu Y, Pan C, Yu G (2021) Building carbazole-decorated styrene–acrylic copolymer latexes and films for iron (III) ion detection. Colloids Surf, A Physicochem Eng Aspects 629:127487
Cheaburn-Yilmaz CN, Yilmaz D, Darie-Nita RN (2021) The effect of different soft core/hard shell ratios on the coating performance of acrylic copolymer latexes. Polymers 13(20):3521
Pakdel AS, Cranston ED, Dube MA (2021) Incorporating hydrophobic cellulose nanocrystals inside latex particles via mini‐emulsion polymerization. Macromol React Eng 15(5):2100023
Cencha LG, Allasia M, Passeggi MC, Gugliotta LM, Minari RJ (2021) Formulation of self-crosslinkable hybrid acrylic/casein latex by tannic acid. Prog Org Coat 159:106413
Schreur-Piet LI, Heuts JP (2021) Controlling the particle size in surfactant-free latexes from ω-propenyl oligomers obtained through catalytic chain transfer polymerization. ACS Appl Polym Mater 3(9):4616–4624
Lei M, Huang W, Sun J, Chen Z, Chen W (2021) Synthesis and characterization of high-temperature self-crosslinking polymer latexes and their application in water-based drilling fluid. Power Technol 389:392–405
Gonzales-Sanchez JF, Fernandez JM, Navarro-Blasco I, Alvarez JI (2021) Improving lime-based rendering mortars with admixtures. Constr Build Mater 271:121887
Barquero A, Leiza JR (2021) Evolution of the film properties of 3‐methacryloxypropyl trimethoxysilane containing waterborne acrylic coatings during storage. J Appl Polym Sci 138(6):49796
Nicolini A, Maciel VG, Neto JD, Braganca SR, Jacobi MM (2021) Rheological behavior of fresh latex polymeric mortar by squeeze-flow technique. Constr Build Mater 267:121175
Li Q, Sun G, Lu Y, Luo S, Gao L (2021) Effects of warm-mix asphalt technologies and modifiers on pavement performance of recycled asphalt binders. J Cleaner Production 282:125435
Onishchenko A, Lapchenko A, Fedorenko O, Bieliatynskyi A (2021) Advances in Intelligent Systems and Computing, 2021, 1258AISC, pp. 104–116
Zarybnicka L, Machotova J, Macova P, Machova D, Viani A (2021) Design of polymeric binders to improve the properties of magnesium phosphate cement. Constr Build Mater 290:123202
Soleimani M, Bagheri E, Mosaddegh P, Fang A, Sadeghi M (2021) Stable waterborne epoxy emulsions and the effect of silica nanoparticles on their coatings properties. Prog Org Coat 156:106250
Dogan-Guner EM, Brownell S, Schueneman GT, Shofner ML, Meredith JC (2021) Enabling zero added-coalescent waterborne acrylic coatings with cellulose nanocrystals. Prog Organ Coat 150:105969
Tessanan WW, Chanthateyanonth R, Yamaguchi M, Phinyocheep P (2020) Improvement of mechanical and impact performance of poly (lactic acid) by renewable modified natural rubber. J Clean Prod 276:123800
Nguyen TH, Tran TT, Kawahava S, Ougizawa T (2020) Preparation of polyaniline nanomatrix formed in natural rubber. Polym J 52(12):1357–1365
Li C, Cheng W, Yan Z, Pan D, Guo Z (2021) Soap-free styrene-acrylic/carbon nanotubes composite latex by in situ emulsion polymerization: preparation, properties and characterizations. Colloids Surf 25:101204
Xu C, Wu W, Zheng Z, Nie J, Chen Y (2021) Strengthened, conductivity-tunable, and low solvent-sensitive flexible conductive rubber films with a Zn2+-crosslinked one-body segregated network. Compos Sci Technol 203:108606
Arakkal A, Aazem I, Honey G, Bhat SG, Sailaja GC (2021) Antibacterial polyelectrolytic chitosan derivatives conjugated natural rubber latex films with minimized bacterial adhesion. J Appl Polym Sci 138(1):49608
Alves JB, Mangia LHR, Sousa-Batista ADJ, Ferraz HC, Pinto JC (2020) Macromol Symp 394(1):2000143
Magida MM, Abou Elfadl A, Nouh SA (2020) Modifications induced in natural rubber due to vinylacetate versatic ester copolymer blend concentration and gamma radiation. Polym Bull 77(12):6319–6332
Xu C, Lin M, Wang X, Lin B, Fu L (2021) Fabrication of high-performance magnetic elastomers by using natural polymer as auxiliary dispersant of Fe3O4 nanoparticles. Comps Part A Appl Sci Manufact 140:106158
Narayanan M, Choi KY (2021) High pressure semibatch emulsion and miniemulsion copolymerization of vinyl acetate and ethylene. J Appl Polym Sci 138(5):49784
Lovell PA, El-Aasser MS (1997) Emulsion polymerization and emulsion polymers. Wiley, New York
van Herk AM (2005) Chemistry and technology of emulsion polymerization. Wiley, New York
Erbil YH (2000) Vinyl acetate emulsion polymerization and copolymerization with acrylic monomers. CRC Press Taylor and Francis, London
Makarewicz E, Tworek M, Zalewska A, Tomaszewska J (2022) Synthesis and testing of latex properties of copolymers of ethyl or butyl acrylate with methyl or butyl methacrylate and methacrylic acid. Polym Int 72(4):397–405
Braun D, Cherdron H, Kern W (1976), Praktikum der makromolekularen organischen chemie, Alfred Hüthig Verlang Heidelberg, Khimia, Moskva
Atkins P, De Paula J, Friedman R (2014) Physical chemistry: quanta, matter, and change. Oxford University Press, USA
Ward JM (1975) Structure and properties of oriented polymers. Applied Sciences, London
Sperling LH (1981) Interpretating polymers networks and related materials. Plenum, New York
Jing M, Gao W, Yin L, Young WS, Ryan P (2020) Characterization of colloidally stabilized latex particles by capillary electrophoresis, In: ACS Symposium Series, vol. 1355, chapter 8, American Chemical Society, New York, pp. 109–124
Sonntag H, Strenge K (1987) Coagulation kinetics and structure. Springer Verlang, Berlin
Jelinski LW (1982) Chain structure and conformations of macromolecules. Academic Press, New York
Barton AF (1983) Handbook of solubility parameters and cohesion parameters Boca Raton. CRC Press Inc, Florida
Burke J (1984) Solubility parameters: theory and application. The American Institute for Conservation, UK
Van Krevelen DW (1972) Properties of polymers correlations with chemical structure. Elsevier Publishing Company, Amsterdam-London-New York
Funding
No funds, grants, or other support was received.
Author information
Authors and Affiliations
Contributions
E.M. and M.T. designed an experimental test setup. M.T., E.M., and A.Z. conducted the experiments. E.M. and J.T. analyzed the results. A.Z. prepared graphs. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Makarewicz, E., Tworek, M., Zalewska, A. et al. Properties of dispersions and films made of copolymers obtained from acrylic, methacrylic and vinyl monomers. Polym. Bull. 81, 6851–6871 (2024). https://doi.org/10.1007/s00289-023-05039-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-023-05039-2