Abstract
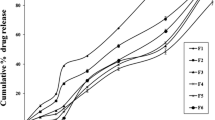
Microbiotically activated ileo-colonic delivery of budesonide from compression coated tablets with considerably reduced carboxymethyl chitosan (CMCH) concentration was designed and investigated. CMCH was synthesized from chitosan dissolved in 20%w/v sodium hydroxide (NaOH) with monochloroacetic at 40 ± 5 °C and characterized by proton NMR, FTIR and Degree of Substitution. The microbiotically activated compression coated tablets (MACC-TABs) were prepared by wet granulation and compression coated with Eudragit S100: HPMC K100M in different ratios for enteric protection and ileo-colon selectivity. Dissolution studies without enzymes and in the presence of enzymes such as pepsin at pH 1.2, diastase and pancreatin at pH 6.8, the F1C3 did not show significant changes. Addition of colonic enzymes at the tenth hour resulted in significant increase in release of budesonide. The % Cumulative Drug Release was 98.49 ± 1.42%, due to the enzymatic triggering (produced by the colonic microflora) leading to the lysis of glycosidic bonds. Scanning electron microscopy revealed the pores formed after dissolution was due to the water-soluble nature of CMCH to release budesonide. The evidence during in-vitro dissolution studies confirmed the pH sensitivity along with microbiotic activation of the MACC-TABs tablets to efficiently and reproducibly release budesonide in the ileo-colon site in the presence of the bacterial enzymes.
Graphic abstract









Similar content being viewed by others
Data availability
The data and supportive information are available within the article, generated through the scientific experiments.
References
John Hopkins University. Crohn's Disease 2001–2013. 600 North Wolfe Street, Baltimore, Maryland 21287. [cited on 11 June 2020] Available from: https://www.hopkinsmedicine.org/gastroenterology_hepatology/_pdfs/small_large_intestine/crohns_disease.pdf
Tremaine WJ (2011) Diagnosis and treatment of indeterminate colitis. Gastroenterol Hepatol (N Y) 7(12):826–828
Francescone R, Hou V, Grivennikov SI, Cytokines IBD (2015) Colitis-associated cancer. Inflamm Bowel Dis 21(2):409–418
Beaugerie L (2014) IBD and increased risk of cancer: what is the reality? Rev Infirm 63(199):28
Stormont JM, Shah AN, Sharma AK, Saubermann LJ, Farmer RG (2013) Colorectal cancer in IBD patients. Am J Gastroenterol 108(9):1535. https://doi.org/10.1038/ajg.2013.203
Ng SC, Shi HY, Hamidi N et al (2018) Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 390(10114):2769–2778. https://doi.org/10.1016/S0140-6736(17)32448-0
Gomollón F, Dignass A, Annese V, Assche HTGV, Lindsay JO (2016) 3rd European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: part 1: diagnosis and medical management. J Crohns Colitis 2017:3–25
Kalla R, Ventham NT, Satsangi J (2014) Crohn’s disease. BMJ 349:g6670
Baumgart DC, Sandborn WJ (2012) Crohn’s disease. Lancet 380:1590–1605
Kaplan GG, Jess T (2016) The changing landscape of inflammatory bowel disease: east meets west. Gastroenterology 150:24–36
Ng SC, Tang W, Ching JY, Wong M, Chow CM, Hui AJ et al (2013) Incidence and phenotype of inflammatory bowel disease based on results from the Asia-Pacific Crohn’s and colitis epidemiology study. Gastroenterology 145:158–165
Sands BE (2004) From symptom to diagnosis: clinical distinctions among various forms of intestinal inflammation. Gastroenterology 126:1518–1532
Jairath V, Feagan BG (2020) Global burden of inflammatory bowel disease. Lancet Gastroenterol Hepatol 5(1):2–3
Desreumaux P, Ghosh S (2006) Review article: mode of action and delivery of 5-aminosalicylic acid - new evidence. Aliment Pharmacol Ther 24(Suppl 1):2–9
Edsbacker S, Andersson T (2004) Pharmacokinetics of budesonide (Entocort EC) capsules for Crohn’s disease. Clin Pharmacokinet 43(12):803–821
Myers B, Evans DN, Rhodes J, Evans BK, Hughes BR, Lee MG et al (1987) Metabolism and urinary excretion of 5-amino salicylic acid in healthy volunteers when given intravenously or released for absorption at different sites in the gastrointestinal tract. Gut 28(2):196–200
Edsbacker S, Bengtsson B, Larsson P, Lundin P, Nilsson A, Ulmius J et al (2003) A pharmacoscintigraphic evaluation of oral budesonide given as controlled-release (Entocort) capsules. Aliment Pharmacol Ther 17(4):525–536
Costa P, Sousa Lobo JM (2001) Modeling and comparison of dissolution profiles. Eur J Pharm Sci 13(2):123–133
Mota FL, Carneiro AP, Queimada AJ, Pinho SP, Macedo EA (2009) Temperature and solvent effects in the solubility of some pharmaceutical compounds: measurements and modeling. Eur J Pharm Sci 37(3–4):499–507
Sinha VR, Mittal BR, Bhutani KK, Kumria R (2004) Colonic drug delivery of 5-fluorouracil: an in vitro evaluation. Int J Pharm 269(1):101–108
Farag RK, Mohamed RR (2012) Synthesis and characterization of carboxymethyl chitosan nanogels for swelling studies and antimicrobial activity. Molecules 18(1):190–203
Vaghani SS, Patel MM, Satish CS (2012) Synthesis and characterization of pH-sensitive hydrogel composed of carboxymethyl chitosan for colon targeted delivery of ornidazole. Carbohydr Res 347:76–82
Abreu F, Campana Filho SP (2005) Preparation and characterization of carboxymethylchitosan. Polímeros Ciência e Tecnologia 15(2):79–83
Capitani D, Porro F, Segre AL (2000) High field NMR analysis of the degree of substitution in carboxymethyl cellulose sodium salt. Carbohydr Polym 42(3):283–286
Zamani A, Henriksson D, Taherzadeh MJ (2010) A new foaming technique for production of superabsorbents from carboxymethyl chitosan. Carbohydr Polym 80:1091–1101
Guzman ML, Marques MR, Olivera ME, Stippler ES (2016) Enzymatic activity in the presence of surfactants commonly used in dissolution media, Part 1: pepsin. Results Pharma Sci 6:15–21
Marques MRC (2014) Enzymes in the dissolution testing of gelatin capsules. AAPS PharmSciTech 15(6):1410–1416
Wahlgren M, Axenstrand M, Håkansson A, Marefati A, Pedersen BL (2019) In vitro methods to study colon release: state of the art and an outlook on new strategies for better in-vitro biorelevant release media. Pharmaceutics 11:95
Yadav S, Deka SR, Tiwari K, Sharma AK, Kumar P (2017) Multi-stimuli responsive self-assembled nanostructures useful for colon drug delivery. IEEE Trans NanoBiosci 16:764–772
Rao J, Khan A (2013) Enzyme sensitive synthetic polymer micelles based on the azobenzene motif. J Am Chem Soc 135(38):14056–14059
Acknowledgements
The authors are thankful to the management of PES University, Bengaluru, India for providing all the necessary facilities and support through a scholarship to one of the authors.
Funding
This research activity was funded by the management of PES University (Grant number: PES1PD19PC005), Bengaluru, India through a scholarship to one of the authors; Nikhil Sutar for conducting this research work.
Author information
Authors and Affiliations
Contributions
All the experimental evidence, article writing, creativity and presentation is a product of the original research work of the first author NS. SCS recommended this topic based on the feasibility of the project and the available resources. He served as the supervisor/guide for this research activity.
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no conflict of interest.
Ethics approval and consent to participate
Not applicable.
Human and animal rights
No animals/humans were used for studies that are basis of this research.
Consent for publication
All the figures/schemes, tables and other illustrations were derived from pre-requisite subject knowledge, the experimental data and are original works of the first author. The graphical abstract was created using bitmap paint skills of the first author. There are no third-party photos, pictures or content used in this research article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sutar, N., Satish, C.S. Design of ileo-colon releasing tablet dosage form by compression coating: effect of carboxymethyl chitosan on budesonide release. Polym. Bull. 81, 2111–2128 (2024). https://doi.org/10.1007/s00289-023-04804-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-023-04804-7