Abstract
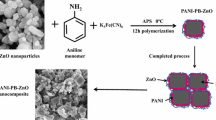
In the present work, PANi/ZnO nanocomposite has been chemically synthesized by using formic acid. PANi/ZnO nanocomposites containing different weight content of ZnO nanoparticles (10, 20 and 30 wt%). ZnO nanoparticles were prepared by the co-precipitation method. Characteristics such as structural, morphological and absorption of the prepared nanocomposites were studied by XRD, FT-IR, SEM and UV–vis spectra. X-ray diffraction of zinc oxide nanoparticles confirmed formation of hexagonal wurtzite crystalline structure, also the substituted zinc oxide nanoparticles in different percentage to semi-crystalline polyaniline indicating nearly the same crystalline structure of PANi/ZnO nanocomposites. The average crystallite size of prepared samples was estimated using XRD, ranging from 11.66 to 25.26 nm, and SEM images revealed an average particle size of 19.72–15.03 nm. The Fourier transform infrared spectra (FT-IR) of ZnO nanoparticles, PANi and their composites have been analyzed in the frequency range between 400 and 4000 cm−1. The characteristic FT-IR peaks of ZnO nanoparticles show shifts to lower wave numbers in all composites. These observed effects may be due to conjugation and some chemical interactions between ZnO nanoparticles and PANI molecular chain. The results confirmed that the PANI successfully grown on surface of ZnO nanoparticles. The ZnO nanoparticles, PANI and PANi/ZnO nanocomposites were further applied to remove methylene orange dye, the effect of pH, initial dye concertation and ZnO nanoparticles dose were investigated via a batch experimental. According to the results, that the hybrid composite of PANi/ZnO increased the photo degradation of methylene orange dye molecules in aqueous solution compared with parent materials. Maximum removal efficiencies at optimum conditions were higher than 97% at pH 11, concentration of dye:10 mg/l, time equal 40 min and dose 0.1 g of 30%ZnO/70%PANi.















Similar content being viewed by others
Abbreviations
- BOD:
-
Biochemical oxygen content
- qe :
-
Adsorption capacity (mg/g)
- C i :
-
Initial concentration (mg L−1)
- C f :
-
Final concentration (mg L−1)
- V :
-
Volume of the solution in (L)
- M :
-
The amount of the adsorbent (g)
- Ce :
-
Concentration of adsorbate at equilibrium state (mg L−1)
- qmax :
-
Langmuir constant relating to adsorption capacity (mg/g)
- b:
-
Constant refers to adsorption energy (L/mg)
- \({K}_{f}\) :
-
Constant related to the adsorption capacity of adsorbent
- n:
-
Constant depends on adsorption intensity value
- q t :
-
The amount of adsorbed dye at time (t) (mg/g)
- K 1 :
-
Pseudo-first-order equilibrium rate constant (1/min)
- K 2 :
-
Pseudo-second-order rate constant (g/mg min)
- MO:
-
Methyl orange dye
References
Cherniwchan J (2012) Economic growth, industrialization, and the environment. Res Energy Econ 34:442–467
Forgacs E, Cserháti T, Oros G (2004) Removal of synthetic dyes from wastewaters: a review. Environ Int 30:953–971
Murugesan K, Kalaichelvan P (2003) Synthetic dye decolourization by white rot fungi. NISCAIR-CSIR, India
Mittal A, Mittal J, Malviya A, Kaur D, Gupta VK (2010) Adsorption of hazardous dye crystal violet from wastewater by waste materials. J Colloid Interface Sci 343:463–473
Deshannavar UB, Ratnamala GM, Kalburgi PB, El-Harbawi M, Agarwal A, Shet M, Teli M, Bhandare P (2016) Optimization, kinetic and equilibrium studies of disperse yellow 22 dye removal from aqueous solutions using malaysian teak wood sawdust as adsorbent. Indian Chem Eng 58:12–28
Sardar M, Manna M, Maharana M, Sen S (2021) Remediation of dyes from industrial wastewater using low-cost adsorbents. Green adsorbents to remove metals dyes and boron from polluted water. Springer, pp 377–403
Gürses A, Açıkyıldız M, Güneş K, Gürses MS (2016) Classification of Dye and Pigments. In: Gürses A, Açıkyıldız M, Güneş K, Gürses MS (eds) Dyes and Pigments. Springer International Publishing, Cham, pp 31–45
Nasar A, Mashkoor F (2019) Application of polyaniline-based adsorbents for dye removal from water and wastewater—a review. Environ Sci Pollut Res 26:5333–5356
Lee J-W, Choi S-P, Thiruvenkatachari R, Shim W-G, Moon H (2006) Evaluation of the performance of adsorption and coagulation processes for the maximum removal of reactive dyes. Dyes Pigm 69:196–203
Raval NP, Shah PU, Shah NK (2016) Adsorptive amputation of hazardous azo dye Congo red from wastewater: a critical review. Environ Sci Pollut Res 23:14810–14853
Yagub MT, Sen TK, Afroze S, Ang HM (2014) Dye and its removal from aqueous solution by adsorption: a review. Adv Coll Interface Sci 209:172–184
Saravanan R, Sacari E, Gracia F, Khan MM, Mosquera E, Gupta VK (2016) Conducting PANI stimulated ZnO system for visible light photocatalytic degradation of coloured dyes. J Mol Liq 221:1029–1033
Saini P (2015) Conjugated polymer-based blends, copolymers, and composites: synthesis, properties, and applications. Wiley, pp 3–118
Monsef Khoshhesab Z, Souhani S (2018) Adsorptive removal of reactive dyes from aqueous solutions using zinc oxide nanoparticles. J Chin Chem Soc 65:1482–1490
Alam M, Alandis NM, Ansari AA, Shaik MR (2013) Optical and electrical studies of polyaniline/ZnO nanocomposite. J Nanomater 2013:157810
Sharma BK, Gupta AK, Khare N, Dhawan SK, Gupta HC (2009) Synthesis and characterization of polyaniline–ZnO composite and its dielectric behavior. Synth Met 159:391–395
Ramakrishnan S, Rajakarthihan S (2020) Low-dose gamma irradiation effect on structural and optical properties of PANI–ZnO composite thin films. Appl Radiat Isot 160:109104
Ahmed F, Kumar S, Arshi N, Anwar MS, Su-Yeon L, Kil G-S, Park D-W, Koo BH, Lee CG (2011) Preparation and characterizations of polyaniline (PANI)/ZnO nanocomposites film using solution casting method. Thin Solid Films 519:8375–8378
Olad A, Asadi N, Mohammadi Aref S, Nosrati R (2018) The use of adsorption method to preparation of polyaniline/ZnO nanocomposite varistor. J Mater Sci Mater Electron 29:9692–9699
Mohsen RM, Morsi SMM, Selim MM, Ghoneim AM, El-Sherif HM (2019) Electrical, thermal, morphological, and antibacterial studies of synthesized polyaniline/zinc oxide nanocomposites. Polym Bull 76:1–21
Ma HC, Zhang Y, Fu YH, Yu CL, Song Y, Ma C, Dong XL (2012) Preparation of polyaniline–ZnO Photocatalyst and its photocatalytic property. Adv Mater Res 356–360:519–523
Lin Y, Wang J, Yang H, Wang L (2017) In situ preparation of PANI/ZnO/CoFe2O4 composite with enhanced microwave absorption performance. J Mater Sci Mater Electron 28:17968–17975
Gilja V, Vrban I, Mandić V, Žic M, Hrnjak-Murgić Z (2018) Preparation of a PANI/ZnO composite for efficient photocatalytic degradation of acid blue. Polymers 10:940
Pandimurugan R, Thambidurai S (2016) Synthesis of seaweed-ZnO-PANI hybrid composite for adsorption of methylene blue dye. J Environ Chem Eng 4:1332–1347
Gabal MA, Al-Juaid AA, El-Rashed S, Hussein MA, Al Angari YM, Saeed A (2019) Structural, thermal, magnetic and electrical properties of polyaniline/CoFe2O4 nano-composites with special reference to the dye removal capability. J Inorg Organomet Polym Mater 29:2197–2213
Alardhi SM, Alrubaye JM, Albayati TM (2020) Adsorption of methyl green dye onto MCM-41: equilibrium, kinetics and thermodynamic studies. Desalin Water Treat 179:323–331
Salman AD, Juzsakova T, Jalhoom MG, Le Phuoc C, Mohsen S, Adnan Abdullah T, Zsirka B, Cretescu I, Domokos E, Stan CD (2020) Novel hybrid nanoparticles: Synthesis, functionalization, characterization, and their application in the uptake of scandium (III) ions from aqueous media. Materials 13:5727
Al-Jadir T, Alardhi SM, Al-Sheikh F, Jaber AA, Kadhim WA, Rahim MHA (2022) Modeling of lead (II) ion adsorption on multiwall carbon nanotubes using artificial neural network and Monte Carlo technique. Chem Eng Commun. https://doi.org/10.1080/00986445.2022.2129622
Mrad M, Chouchene B, Chaabane TB (2018) Effects of zinc precursor, basicity and temperature on the aqueous synthesis of ZnO nanocrystals, South African. J Chem 71:103–110
VK Anand, S Sood, A Sharma (2010) Characterization of ZnO thin film deposited by sol–gel process. In: AIP conference proceedings, American Institute of Physics. pp 399–401
Turkten N, Karatas Y, Bekbolet M (2021) Preparation of PANI modified ZnO composites via different methods: structural, morphological and photocatalytic properties. Water 13:1025
Abdalsalam AH, Ati AA, Abduljabbar A, Hussein TA (2019) Structural, optical, electrical and magnetic studies of pani/ferrite nanocomposites synthesized by PLD technique. J Inorg Organomet Polym Mater 29:1084–1093
Salih S, Algabban A, Abdalsalam A (2017) Preparation and characterization of PMMA-HDPE and HDPE-PMMA binary polymer blends. Eng Technol J 35:311–317
Ati MA, Khudhair H, Dabagh S, Rosnan R, Ati AA (2014) Synthesis and characterization of cobalt doped nickel-ferrites nanocrystalline by co-precipitation method. Int J Sci Eng Res 9:927–930
Ati AA, Abdalsalam AH, Hasan AS (2021) Thermal, microstructural and magnetic properties of manganese substitution cobalt ferrite prepared via co-precipitation method. J Mater Sci: Mater Electron 32:3019–3037
Langford J (1975) X-ray diffraction procedures for polycrystalline and amorphous materials by HP Klug and LE Alexander. J Appl Crystallogr 8:573–574
Alam M, Alandis NM, Ansari AA, Shaik MR (2013) Optical and electrical studies of polyaniline/ZnO nanocomposite. J Nanomater 2013:1–5
Ati AA (2018) Fast synthesis, structural, morphology with enhanced magnetic properties of cobalt doped nickel ferrite nanoscale. J Mater Sci: Mater Electron 29:12010–12021
Kayani ZN, Iqbal M, Riaz S, Zia R, Naseem S (2016) Fabrication and properties of zinc oxide thin film prepared by sol–gel dip coating method. Mater Sci-Pol 33:515–520
Shah SS, Sharma T, Dar BA, Bamezai RK (2021) Adsorptive removal of methyl orange dye from aqueous solution using populous leaves: Insights from kinetics, thermodynamics and computational studies. Environ Chem Ecotoxicol 3:172–181
Rápó E, Tonk S (2021) Factors affecting synthetic dye adsorption; desorption studies: a review of results from the last 5 years (2017–2021). Molecules (Basel, Switzerland) 26:5419
Keller AA, Wang H, Zhou D, Lenihan HS, Cherr G, Cardinale BJ, Miller R, Ji Z (2010) Stability and aggregation of metal oxide nanoparticles in natural aqueous matrices. Environ Sci Technol 44:1962–1967
Lei C, Pi M, Jiang C, Cheng B, Yu J (2017) Synthesis of hierarchical porous zinc oxide (ZnO) microspheres with highly efficient adsorption of Congo red. J Colloid Interface Sci 490:242–251
Mahmoud ME, Abou Kana MTH, Hendy AA (2015) Synthesis and implementation of nano-chitosan and its acetophenone derivative for enhanced removal of metals. Int J Biol Macromol 81:672–680
Pooresmaeil M, Mansoori Y, Mirzaeinejad M, Khodayari A (2018) Efficient removal of methylene blue by novel magnetic hydrogel nanocomposites of poly(acrylic acid). Adv Polym Technol 37:262–274
Huang R, Liu Q, Huo J, Yang B (2017) Adsorption of methyl orange onto protonated cross-linked chitosan. Arab J Chem 10:24–32
Jain M, Garg VK, Kadirvelu K (2010) Adsorption of hexavalent chromium from aqueous medium onto carbonaceous adsorbents prepared from waste biomass. J Environ Manage 91:949–957
Boeva ZA, Sergeyev VG (2014) Polyaniline: synthesis, properties, and application. Polym Sci Ser C 56:144–153
Jiang Y, Liu Z, Zeng G, Liu Y, Shao B, Li Z, Liu Y, Zhang W, He Q (2018) Polyaniline-based adsorbents for removal of hexavalent chromium from aqueous solution: a mini review. Environ Sci Pollut Res 25:6158–6174
Mohammadi Nodeh MK, Gabris MA, Rashidi Nodeh H, Esmaeili Bidhendi M (2018) Efficient removal of arsenic (III) from aqueous media using magnetic polyaniline-doped strontium–titanium nanocomposite. Environ Sci Pollut Res 25:16864–16874
Mahmoud ME, Saad EA, El-Khatib AM, Soliman MA, Allam EA, Fekry NA (2018) Green solid synthesis of polyaniline-silver oxide nanocomposite for the adsorptive removal of ionic divalent species of Zn/Co and their radioactive isotopes 65Zn/60Co. Environ Sci Pollut Res 25:22120–22135
Yang G, Wu L, Xian Q, Shen F, Wu J, Zhang Y (2016) Removal of congo red and methylene blue from aqueous solutions by vermicompost-derived biochars. PLoS ONE 11:e0154562
Deng H, Lu J, Li G, Zhang G, Wang X (2011) Adsorption of methylene blue on adsorbent materials produced from cotton stalk. Chem Eng J 172:326–334
Hanifa F, Ehsana S, Zafara S, Akhtara M, Imran M, Manzoorf S (2020) Adsorptive removal of methyl orange and acid blue-2445 from binary system by anion exchange membrane BI: non-linear and linear form of isotherms. Desalin Water Treat 194:290–301
Ahmad A, Rafatullah M, Sulaiman O, Ibrahim MH, Hashim R (2009) Scavenging behaviour of meranti sawdust in the removal of methylene blue from aqueous solution. J Hazard Mater 170:357–365
Banerjee S, Chattopadhyaya MC (2017) Adsorption characteristics for the removal of a toxic dye, tartrazine from aqueous solutions by a low cost agricultural by-product. Arab J Chem 10:S1629–S1638
Acknowledgments
The author would like to thank the Nanotechnology and Advanced Materials Center of University of Technology-Iraq and Institute for Fundamental Science Studies and physics department of Universiti Teknologi Malaysia (UTM) for facilities supports.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alardhi, S.M., Abdalsalam, A.H., Ati, A.A. et al. Fabrication of polyaniline/zinc oxide nanocomposites: synthesis, characterization and adsorption of methylene orange. Polym. Bull. 81, 1131–1157 (2024). https://doi.org/10.1007/s00289-023-04753-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-023-04753-1