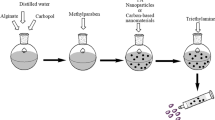
Abstract
A major challenge in treating burn wounds is bacteria invasion which prolongs the wound healing process. Most of the available wound dressings used to treat burn wounds can cause further skin tears upon removal. Due to this limitation, there is a need to design wound dressings that can be easily removed without causing skin tears with desirable antibacterial activity and absorption capability. Dissolvable wound dressings were prepared from a combination of sodium alginate and Poloxamer-407. B02, B08, and B13 loaded with a combination of ZnO NPs and ciprofloxacin or ciprofloxacin alone exhibit promising antibacterial activity against Enterococcus faecalis, Staphylococcus aureus, Proteus mirabilis, Echerischia coli, and Klebsiella aeruginosa. Most of the wound dressings displayed excellent porosity with a rapid uptake of the simulated wound exudate in the range of 870–4468% followed by dissolution. Haemostasis evaluation on the wound dressings reveal low absorbance values of 0.2511 (p-value = 0.0094), 0.2066 (p-value = 0.0002) for B02 and B03 compared to the control (whole blood) suggesting ideal clotting capability. The exceptional features of the prepared wound dressings reveal their potential use for the management of burn wounds on sensitive skin.











Similar content being viewed by others
References
Zhu Y, Zhang J, Song J, Yang J, Xu T, Pan C, Zhang L (2017) One-step synthesis of an antibacterial and pro-healing wound dressing that can treat wound infections. J Mater Chem B 5:8451–8458
Tom IM (2019) Infection of wounds by potential bacterial pathogens and their resistogram. Open Access Libr 6:1–13
Jahromi MA, Zangabad PS, Basri SM, Zangabad KS, Ghamarypour A, Aref AR, Karimi M, Hamblin MR (2018) Nanomedicine and advanced technologies for burns: preventing infection and facilitating wound healing. Adv Drug Deliv Rev 123:33–64
Blanchette KA, Wenke JC (2018) Current therapies in treatment and prevention of fracture wound biofilms: why a multifaceted approach is essential for resolving persistent infections. J Bone Joint Infect 3:50–67
Harats M, Ofir H, Segalovich M, Visentin D, Givon A, Peleg K, Kornhaber R, Cleary M, Haik J (2019) Trends, and risk factors for mortality in elderly burns patients: a retrospective review. Burns 45:1342–1349
Boissin C, Wallis LA, Kleintjes W, Laflamme L (2019) Admission factors associated with the in-hospital mortality of burns patients in resource-constrained settings: a two-year retrospective investigation in a South African adult burns centre. Burns 45:1462–1470
Church D, Elsayed S, Reid O, Winston B, Lindsay R (2006) Burn wound infections. Am Soc Microbiol 19:403–434
Hall CW, Mah TF (2017) Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol Rev 41:276–301
Sharma D, Misba L, Khan AU (2019) Antibiotics versus biofilm: an emerging battleground in microbial communities. Antimicrob Resist Infect Control 8:1–10
Summa M, Russo D, Penna I, Margaroli N, Bayer IS, Bandiera T, Athanassiou A, Bertorelli R (2018) A biocompatible sodium alginate/povidone iodine film enhances wound healing. Eur J Pharm Biopharm 122:17–24
Rowan MP, Cancio LC, Elster EA, Burmeister DM, Rose LF, Natesan S, Chan RK, Christy RJ, Chung KK (2015) Burn wound healing and treatment: review and advancements. Crit Care 19:1–2
Arshad R, Sohail MF, Sarwar HS, Saeed H, Ali I, Akhtar S, Hussain SZ, Afzal I, Jahan S, Shahnaz G (2019) ZnO-NPs embedded biodegradable thiolated bandage for postoperative surgical site infection: in vitro and in vivo evaluation. PLoS ONE 14:e0217079
Rohani RE, Vahabi H, Formela K, Saeb MR, Thomas S (2019) Injectable poloxamer/graphene oxide hydrogels with well-controlled mechanical and rheological properties. Polym Adv Technol 30:2250–2260
Wang T, Wang J, Wang R, Yuan P, Fan Z, Yang S (2019) Preparation and properties of ZnO/sodium alginate bi-layered hydrogel films as novel wound dressings. New J Chem 43:8684–8693
Naseri S, Lepry WC, Nazhat SN (2017) Bioactive glasses in wound healing: hope or hype. J Mater Chem B 5:6167–6174
Chouhan N (2021) Silver nanoparticles: synthesis, characterization and applications, silver nanoparticles, fabrication, characterization and applications. https://www.intechopen.com/chapters/61218, 2021. Accessed 01 January 2021
Batool M, Khurshid S, Qureshi Z, Daoush WM (2021) Adsorption, antimicrobial and wound healing activities of biosynthesised zinc oxide nanoparticles. Chem Pap 75:893–907
Kim M (2017) Nanoparticle-based therapies for wound biofilm infection: opportunities and challenges. IEEE Trans Nanobioscience 15:294–304
Viton C, Agay D, Mari E, Roger T, Chancerelle Y, Domard A (2007) The use of physical hydrogels of chitosan for skin regeneration following third-degree burns. Biomaterials 28:3478–3488
Kwon OJ, Oh ST, Lee SD, Lee NR, Shin CH, Park JS (2007) Hydrophilic and flexible polyurethane foams using sodium alginate as polyol: Effects of PEG molecular weight and cross-linking agent content on water absorbency. Fibers Polym 8:347–355
Mu X, Yu H, Zhang C, Chen X, Cheng Z, Bai R, Wu X, Yu Q, Wu C, Diao Y (2016) Nano-porous nitrocellulose liquid bandage modulates cell and cytokine response and accelerates cutaneous wound healing in a mouse model. Carbohydr Polym 136:618–629
Zhao Y, Li Z, Li Q, Yang L, Liu H, Yan R, Xiao L, Liu H, Wang J, Yang B, Lin Q (2020) Transparent conductive supramolecular hydrogels with stimuli-responsive properties for on-demand dissolvable diabetic foot wound dressings. Macromol Rapid Comm 41:1–11
Huang W, Wang Y, Huang Z, Wang X, Chen L, Zhang Y, Zhang L (2018) On-demand dissolvable self-healing hydrogel based on carboxymethyl chitosan and cellulose nanocrystal for deep partial thickness burn wound healing. ACS Appl Mater Interfaces 10:41076–41088
Ghobril C, Charoen K, Rodriguez EK, Nazarian A, Grinstaff MW (2013) A dendritic thioester hydrogel based on thiol–thioester exchange as a dissolvable sealant system for wound closure. Angew Chem Int Ed 52:14070–14074
Hassabo AA, Ibrahim EI, Ali BA, Emam HE (2022) Anticancer effects of biosynthesized Cu2O nanoparticles using marine yeast. Biocatal Agric Biotechnol 39:10226
Ahmed HB, Emam HE (2021) Overview for multimetallic nanostructures with biomedical, environmental and industrial applications. J Mol Liq 321:114669
Ahmed HB, Abdel-Mohsen AM, Emam HE (2016) Green-assisted tool for nanogold synthesis based on alginate as a biological macromolecule. RSC Adv 6(78):73974–73985
Aslan C, Çelebi N, De IT (2017) Development of interleukin-2 loaded chitosan-based nanogels using artificial neural networks and investigating the effects on wound healing in Rats. AAPS PharmSciTech 18:1019–1030
Ngamviriyavong P, Thananuson A, Pankongadisak P, Tanjak P, Janvikul W (2010) Antibacterial hydrogels from chitosan derivatives. J Met Mater Miner 20:113–117
Lin P, Sermersheim M, Li H, Lee PHU, Steinberg SM, Ma J (2018) Zinc in wound healing modulation. Nutrients 10(1):16
Liu H, Wang C, Li C, Qin Y, Wang Z, Yang F, Li Z, Wang J (2018) A functional chitosan-based hydrogel as a wound dressing and drug delivery system in the treatment of wound healing. RSC Adv 8:7533–7549
Naghshineh N, Tahvildari K, Nozari M (2019) Preparation of chitosan, sodium alginate, gelatin and collagen biodegradable sponge composites and their application in wound healing and curcumin delivery. J Polym Environ 27:2819–2830
Francolini I, Vuotto C, Piozzi A, Donelli G (2017) Antifouling and antimicrobial biomaterials: an overview. APMIS 125:392–417
Okur ME, Ayla S, Batur S, Yoltas Y, Genc E, Pertek S, Okuret NU (2019) Evaluation of in situ gel containing pycnogenol for cutaneous wound healing. Medeni Med J 34:67–75
Sharma N, Jandaik S, Kumar S (2016) Synergistic activity of doped zinc oxide nanoparticles with antibiotics: ciprofloxacin, ampicillin, fluconazole and amphotericin B against pathogenic microorganisms. An Acad Bras Cienc 88:1689–1698
Ye H, Cheng J, Yu K (2019) In situ reduction of silver nanoparticles by gelatin to obtain porous silver nanoparticle/chitosan composites with enhanced antimicrobial and wound-healing activity. Int J Biol Macromol 121:633–642
Akiyode O, Boateng J (2018) Composite biopolymer-based wafer dressings loaded with microbial biosurfactants for potential application in chronic wounds. Polymers (Basel) 10:918
Buyana B, Aderibigbe BA, Ray SS, Ndinteh DT, Fonkui YT (2019) Development, characterization, and in vitro evaluation of water soluble poloxamer/pluronic-mastic gum-gum acacia-based wound dressing. J Appl Polym Sci 48728:1–10
Pan H, Fan D, Cao W, Zhu C, Duan Z, Fu R, Li X, Ma X (2017) Preparation and characterization of breathable haemostatic hydrogel dressings and determination of their effects on full-thickness defects. Polymers 9:727
Fonkui TY, Ikhile MI, Njobeh PB, Ndinteh DT (2019) Benzimidazole Schiff base derivatives: synthesis, characterization and antimicrobial activity. BMC Chem 13:127
Catanzano O, D’Esposito V, Formisano P, Boateng JS, Quaglia F (2018) Composite alginate-hyaluronan sponges for the delivery of tranexamic acid in postextractive alveolar wounds. J Pharm 107:654–661
Pandey S, Pandey P, Tiwari G, Tiwari R, Rai AK (2012) FTIR spectroscopy: a tool for quantitative analysis of ciprofloxacin in tablets. Indian J Pharm Sci 74:86
Mehrabani MG, Karimian R, Mehramouz B, Rahimi M, Kafil HS (2018) Preparation of biocompatible and biodegradable silk fibroin/chitin/silver nanoparticles 3D scaffolds as a bandage for antimicrobial wound dressing. Int J Biol Macromol 114:961–971
Demirbilek M, Türkoğlu LN, Aktürk S (2017) N-acetylglucoseamine modified alginate sponges as scaffolds for skin tissue engineering. Turkish J Biol 41:796–807
Rezvani GE, Khalili S, Nouri KS, Esmaeely NR, Ramakrishna S (2019) Wound dressings: current advances and future directions. J Appl Polym Sci 136:1–12
Hu Y, Zhang Z, Li Y, Ding X, Li D, Shen C, Xu FJ (2018) Dual-crosslinked amorphous polysaccharide hydrogels based on chitosan/alginate for wound healing applications. Macromol Rapid Commun 39:1–5
Rezvanian M, Tan CK, Ng SF (2016) Simvastatin-loaded lyophilized wafers as a potential dressing for chronic wounds. Drug Dev Ind Pharm 42:2055–2062
Pelegrino MT, Lima BDA, do Nascimento MHM, Lombello CB, Brocchi M, Seabra AB (2018) Biocompatible and antibacterial nitric oxide-releasing pluronic F-127/Chitosan hydrogel for topical applications. Polymers (Basel) 10:1–20
Li X, Ma M, Uk D, Huang X (2019) Preparation and characterization of novel eggshell membrane-chitosan blend fi lms for potential wound-care dressing: from waste to medicinal products. Int J Biol Macromol 123:477–484
Poonguzhali R, Basha SK, Kumari VS (2018) Novel asymmetric chitosan/PVP/nanocellulose wound dressing: in vitro and in vivo evaluation. Int J Biol Macromol 112:1300–1309
Amini MM, Di MA, Šopík T, Fei H, Císař J, Pummerová M, Sedlařík V (2021) Polylactide/polyvinylalcohol-based porous bioscaffold loaded with gentamicin for wound dressing applications. Polymers 13:921
Homaeigohar S, Boccaccini AR (2020) Antibacterial biohybrid nanofibers for wound dressings. Acta Biomater 15:25–49
Andreu V, Mendoza G, Arruebo M, Irusta S (2015) Smart dressings based on nanostructured fibers containing natural origin antimicrobial, anti-inflammatory, and regenerative compounds. Mater 8:5154–5193
Sk PT, Lakshmanan VK, Raj M, Biswas R, Hiroshi T, Nair SV, Jayakumar R (2013) Evaluation of wound healing potential of β-chitin hydrogel/nano zinc oxide composite bandage. Pharm Res 30(2):523–537
Ahmed A, Boateng J (2018) Calcium alginate-based antimicrobial film dressings for potential healing of infected foot ulcers. Ther Deliv 9:185–204
Li Y, Jiang H, Zheng W, Gong N, Chen L, Jiang X, Yang G (2015) Bacterial cellulose–hyaluronan nanocomposite biomaterials as wound dressings for severe skin injury repair. J Mater Chem B 3:3498–3507
Chen SH, Tsao CT, Chang CH, Lai YT, Wu MF, Chuang CN, Chou HC, Wang CK, Hsieh KH (2013) Assessment of reinforced poly (ethylene glycol) chitosan hydrogels as dressings in a mouse skin wound defect model. Mater Sci Eng 33:2584–2594
Shrimali H, Mandal UK, Nivsarkar M, Shrivastava N (2019) Fabrication and evaluation of a medicated hydrogel film with embelin from Embelia ribes for wound healing activity. Future J Pharm Sci 5:1–10
Ujang Z, Rashid AH, Suboh SK, Halim AS, Lim CK (2014) Physical properties and biocompatibility of oligochitosan membrane film as wound dressing. J Appl Biomater Funct Mater 12:155–162
Steffy K, Shanthi G, Maroky AS, Selvakumar S (2018) Enhanced antibacterial effects of green synthesized ZnO NPs using Aristolochia indica against Multi-drug resistant bacterial pathogens from Diabetic Foot Ulcer. J Infect Public Health 11:463–471
Massarelli E, Silva D, Pimenta AF, Fernandes AI, Mata JL, Armês H, Salema-Oom M, Saramago B, Serro AP (2021) Polyvinyl alcohol/chitosan wound dressings loaded with antiseptics. Int J Pharm 25:120110
Qu J, Zhao X, Liang Y, Xu Y, Ma PX, Guo B (2019) Degradable conductive injectable hydrogels as novel antibacterial, antioxidant wound dressings for wound healing. Chem Eng J 15:548–560
Kumar M, Sura JPAL (2021) Mastic-g-poly (acrylamide): microwave-assisted synthesis and characterization. Int J App Pharm 13(2):228–234
Mathur G, Prasad R (2012) Degradation of polyurethane by Aspergillus flavus (ITCC 6051) isolated from soil. Appl Biochem Biotechnol 167(6):1595–1602
Zulkifli FH, Hussain FS, Rasad MS, Yusoff MM (2014) In vitro degradation study of novel HEC/PVA/collagen nanofibrous scaffold for skin tissue engineering applications. Polym Degrad Stab 110:473–481
Zhou J, Cao C, Ma X, Hu L, Chen L, Wang C (2010) In vitro and in vivo degradation behavior of aqueous-derived electrospun silk fibroin scaffolds. Polym Degrad Stab 95(9):1679–1685
Wang HM, Chou YT, Wen ZH, Wang ZR, Chen CH, Ho ML (2013) Novel biodegradable porous scaffold applied to skin regeneration. PLoS ONE 8(6):e56330
Mogoşanu GD, Grumezescu AM (2014) Natural and synthetic polymers for wounds and burns dressing. Int J Pharm 463(2):127–136
Fontecha-Umaña F, Ríos-Castillo AG, Ripolles-Avila C, Rodríguez-Jerez JJ (2020) Antimicrobial activity, and prevention of bacterial biofilm formation of silver and zinc oxide nanoparticle-containing polyester surfaces at various concentrations for use. Foods 9:1–15
Abo-Shama UH, El-Gendy H, Mousa WS, Hamouda RA, Yousuf WE, Hetta HF, Abdeen EE (2020) Synergistic and antagonistic effects of metal nanoparticles in combination with antibiotics against some reference strains of pathogenic microorganisms. Infect Drug Resist 13:351–362
Dizaj SM, Lotfipour F, Barzegar-Jalali M, Zarrintan MH, Adibkia K (2014) Antimicrobial activity of the metals and metal oxide nanoparticles. Mater Sci Eng C Mater Biol Appl 44:278–284
Sirelkhatim A, Mahmud S, Seeni A (2015) Review on zinc oxide nanoparticles: antibacterial activity and toxicity mechanism. NanoMicro Lett 7:219–242
Agarwal H, Menon S, Kumar SV, Rajeshkumar S (2018) Mechanistic study on antibacterial action of zinc oxide nanoparticles synthesized using green route. Chem Biol Interact 286:60–70
Raghupathi KR, Koodali RT, Manna AC (2011) Size-dependent bacterial growth inhibition and mechanism of antibacterial activity of zinc oxide nanoparticles. Langmuir 27:4020–4028
Kadiyala U, Turali-Emre ES, Bahng JH, Kotov NA, VanEpps JS (2018) Unexpected insights into antibacterial activity of zinc oxide nanoparticles against methicillin resistant Staphylococcus aureus (MRSA). Nanoscale 10:4927–4939
Pillai AM, Sivasankarapillai VS, Rahdar A, Joseph J, Sadeghfar F, Rajesh K, Kyzas GZ (2020) Green synthesis and characterization of zinc oxide nanoparticles with antibacterial and antifungal activity. J Mol Struct 1211:128107
Serra R, Grande R, Butrico L, Rossi A, Settimio UF, Caroleo B, Amato B, Gallelli L, Franciscis S (2015) Chronic wound infections: the role of Pseudomonas aeruginosa and Staphylococcus aureus. Exp Rev Anti-infect Ther 13:605–613
Chong KK, Tay WH, Janela B, Yong AM, Liew TH, Madden L, Keogh D, Barkham TM, Ginhoux F, Becker DL, Kline KA (2017) Enterococcus faecalis modulates immune activation and slows healing during wound infection. J Infect Dis 16:1644–1654
Sharma L, Srivastava H, Pipal DK, Dhawan R, Purohit PM, Bhargava A (2017) Bacteriological profile of burn patients and antimicrobial susceptibility pattern of burn wound isolates. Int Surg J 4:1019–1023
Lindsay S, Oates A, Bourdillon K (2017) The detrimental impact of extracellular bacterial proteases on wound healing. Int Wound J 14:1237–1247
Gupta D, Singh A, Khan AU (2017) Nanoparticles as efflux pump and biofilm inhibitor to rejuvenate bactericidal effect of conventional antibiotics. Nanoscale Res Lett 12:1–6
Timmons J (2009) Alginates as haemostatic agents: worth revisiting. Wounds UK 5:122–125
Steuer H, Krastev R, Lembert N (2014) Metallic oxide nanoparticles stimulate blood coagulation independent of their surface charge. J Biomed Mater Res 102:897–902
Shefa AA, Taz M, Hossain M, Kim YS, Lee SY, Lee BT (2019) Investigation of efficiency of a novel, zinc oxide loaded TEMPO-oxidized cellulose nanofiber-based hemostat for topical bleeding. Int J Biol Macromol 126:786–795
Yang JY, Bae J, Jung A, Park S, Chung S, Seok J, Roh H, Han Y, Oh JM, Sohn S, Jeong J (2017) Surface functionalization-specific binding of coagulation factors by zinc oxide nanoparticles delays coagulation time and reduces thrombin generation potential in vitro. PLoS ONE 12:0181634
Mohandas A (2020) Exploration of alginate hydrogel/nano zinc oxide composite bandages for infected wounds. Polym J 2020:53–66
Acknowledgements
The financial support of Govan Mbeki Research and Development Centre, University of Fort Hare, South Africa Medical Research Council, Sasol Foundation, and National Research Foundation, South Africa towards this research is hereby acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors hereby declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ndlovu, S.P., Fonkui, T.Y., Kumar, P. et al. Dissolvable zinc oxide nanoparticle-loaded wound dressing with preferential exudate absorption and hemostatic features. Polym. Bull. 80, 7491–7518 (2023). https://doi.org/10.1007/s00289-022-04358-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-022-04358-0