Abstract
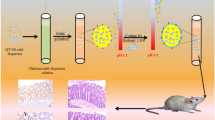
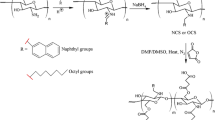
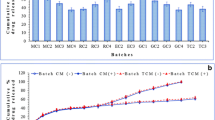
The aim of the present study was to synthesize the chitosan-encapsulated daidzein (CED) and to evaluate its releasing behaviour in the different simulated gastrointestinal fluids. Moreover, the cytotoxic potential and antiangiogenic effects of CED were determined against human colon cancer cells and chorioallantoic blood vessels, respectively. The synthesized daidzein-loaded microcapsules possessed micrometre size, with good stability in colloidal dispersion with 64.3% entrapment efficiency. The release behaviour assay indicated the gradual release of daidzein in the simulated stomach and intestinal fluid (~ 38%; pH 1.2–4.5), while the substantial release was occurred in the simulated colonic fluid (79%; pH 6.8–7). The daidzein-loaded microcapsules inhibited the proliferation of human colorectal cancer cells (HT-29) with no prominent reduction in the viability of human dermal fibroblast (HDF). The expression analysis of caspase-3 gene together with flow cytometry results confirmed the apoptotic cells death in the HT-29 cells induced by daidzein-loaded microcapsules. Further, the daidzein-loaded microcapsules indicated the angiogenesis inhibitory activity. Consequently, these results revealed that microencapsulation of daidzein by chitosan could be a feasible approach for the delivery of daidzein, a soy-derived isoflavone for the colon cancer prevention and treatment.








Similar content being viewed by others
References
Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F (2017) Global patterns and trends in colorectal cancer incidence and mortality. Gut 66(4):683–691
Porter AG, Jänicke RU (1999) Emerging roles of caspase-3 in apoptosis. Cell Death Differ 6(2):99–104
Cairns R HI, McCracken S, Mak T (2011) Cancer cell metabolism. In: Cold Spring Harbor symposia on quantitative biology. New York: Cold Spring Harbor Laboratory Press: 299–311.
Nishida N, Yano H, Nishida T, Kamura T, Kojiro M (2006) Angiogenesis in cancer. Vascular Health Risk Manag 2(3):213
Afrin S, Giampieri F, Gasparrini M, Forbes-Hernández TY, Cianciosi D, Reboredo-Rodriguez P, Zhang J, Manna PP, Daglia M, Atanasov AG (2020) Dietary phytochemicals in colorectal cancer prevention and treatment: a focus on the molecular mechanisms involved. Biotechnol Adv 38:107322
Akhtar Siddiqui J, Singh A, Chagtoo M, Singh N, Madhav Godbole M, Chakravarti B (2015) Phytochemicals for breast cancer therapy: current status and future implications. Curr Cancer Drug Targets 15(2):116–135
Meybodi NM, Mortazavian AM, Monfared AB, Sohrabvandi S, Meybodi FA (2017) Phytochemicals in cancer prevention: a review of the evidence. Iran J Cancer Prevent, 10(1).
Hosseini A, Ghorbani A (2015) Cancer therapy with phytochemicals: evidence from clinical studies. Avicenna J Phytomed 5(2):84
Cassidy, (2003) Potential risks and benefits of phytoestrogen-rich diets. Int J Vitam Nutr Res 73(2):120–126
Wang X, Wu J, Chiba H, Umegaki K, Yamada K, Ishimi Y (2003) Puerariae radix prevents bone loss in ovariectomized mice. J Bone Miner Metab 21(5):268–275
Wong KH, Li GQ, Li KM, Razmovski-Naumovski V, Chan K (2011) Kudzu root: traditional uses and potential medicinal benefits in diabetes and cardiovascular diseases. J Ethnopharmacol 134(3):584–607
Jaiswal M, Dudhe R, Sharma P (2015) Nanoemulsion: an advanced mode of drug delivery system. 3 Biotech 5(2):123–127
Zabielska-Koczywąs K, Lechowski R (2017) The use of liposomes and nanoparticles as drug delivery systems to improve cancer treatment in dogs and cats. Molecules 22(12):2167
Li J, Cai C, Li J, Li J, Li J, Sun T, Wang L, Wu H, Yu G (2018) Chitosan-based nanomaterials for drug delivery. Molecules 23(10):2661
Mitra A, Dey B (2011) Chitosan microspheres in novel drug delivery systems. Indian J Pharm Sci 73(4):355
Konecsni K, Low N, Nickerson M (2012) Chitosan–tripolyphosphate submicron particles as the carrier of entrapped rutin. Food Chem 134(4):1775–1779
Crozier A, Jensen E, Lean ME, McDonald MS (1997) Quantitative analysis of flavonoids by reversed-phase high-performance liquid chromatography. J Chromatogr A 761(1–2):315–321
Zhang L, Kosaraju SL (2007) Biopolymeric delivery system for controlled release of polyphenolic antioxidants. Eur Polymer J 43(7):2956–2966
Laird BD, Van de Wiele TR, Corriveau MC, Jamieson HE, Parsons MB, Verstraete W, Siciliano SD (2007) Gastrointestinal microbes increase arsenic bioaccessibility of ingested mine tailings using the simulator of the human intestinal microbial ecosystem. Environ Sci Technol 41(15):5542–5547
Sanaeimehr Z, Javadi I, Namvar F (2018) Antiangiogenic and antiapoptotic effects of green-synthesized zinc oxide nanoparticles using Sargassum muticum algae extraction. Cancer nanotechnol 9(1):1–16
Salopek B, Krasic D, Filipovic S (1992) Measurement and application of zeta-potential. Rudarsko-geolosko-naftni zbornik 4(1):147
Othman N, Masarudin MJ, Kuen CY, Dasuan NA, Abdullah LC (2018) Synthesis and optimization of chitosan nanoparticles loaded with L-ascorbic acid and thymoquinone. Nanomaterials 8(11):920
Han B-J, Li W, Jiang G-B, Lai S-H, Zhang C, Zeng C-C, Liu Y-J (2015) Effects of daidzein in regards to cytotoxicity in vitro, apoptosis, reactive oxygen species level, cell cycle arrest and the expression of caspase and Bcl-2 family proteins. Oncol Rep 34(3):1115–1120
Ruan L, Ge M, Huang X, Ren J (2018) Assay of single-cell apoptosis by ensemble and single-molecule fluorescence methods: annexin-V/polyethylene glycol-functionalized quantum dots as probes. Langmuir 34(34):10040–10047
Verma M, Singh SK, Bhushan S, Pal HC, Kitchlu S, Koul MK, Thappa RK, Saxena AK (2008) Induction of mitochondrial-dependent apoptosis by an essential oil from tanacetum gracile. Planta Med 74(05):515–520
Wlodkowic D, Skommer J, Darzynkiewicz Z (2009) Flow cytometry-based apoptosis detection. In: Erhardt P, Toth A (eds) apoptosis. Humana Press Springer, pp 19–32
Kessel D, Oleinick NL (2018) Cell death pathways associated with photodynamic therapy: an update. Photochem Photobiol 94(2):213–218
Wang S, Long S, Xiao S, Wu W, Hann SS (2018) Decoction of Chinese herbal medicine Fuzheng Kang-Ai induces lung cancer cell apoptosis via STAT3/Bcl-2/caspase-3 pathway. Evidence-Based Complem Alternat Med 2018:1–14
Kumari A, Singla R, Guliani A, Yadav SK (2014) Nanoencapsulation for drug delivery. EXCLI J 13:265
Agnihotri SA, Mallikarjuna NN, Aminabhavi TM (2004) Recent advances on chitosan-based micro-and nanoparticles in drug delivery. J Control Release 100(1):5–28
Danielsen S, Strand S, de Lange Davies C, Stokke BT (1721) Glycosaminoglycan destabilization of DNA–chitosan polyplexes for gene delivery depends on chitosan chain length and GAG properties. Biochimica et Biophysica Acta (BBA) General Subjects, 1721(1–3): 44–54 Doi: https://doi.org/10.1016/j.bbagen.2004.10.011
Di Martino A, Sittinger M, Risbud MV (2005) Chitosan: a versatile biopolymer for orthopaedic tissue-engineering. Biomaterials 26(30):5983–5990
Ishihara M, Nakanishi K, Ono K, Sato M, Kikuchi M, Saito Y, Yura H, Matsui T, Hattori H, Uenoyama M (2002) Photocrosslinkable chitosan as a dressing for wound occlusion and accelerator in healing process. Biomaterials 23(3):833–840
Madihally SV, Matthew HW (1999) Porous chitosan scaffolds for tissue engineering. Biomaterials 20(12):1133–1142
Chen M-C, Mi F-L, Liao Z-X, Hsiao C-W, Sonaje K, Chung M-F, Hsu L-W, Sung H-W (2013) Recent advances in chitosan-based nanoparticles for oral delivery of macromolecules. Adv Drug Deliv Rev 65(6):865–879
Barbieri S, Buttini F, Rossi A, Bettini R, Colombo P, Ponchel G, Sonvico F, Colombo G (2015) Ex vivo permeation of tamoxifen and its 4-OH metabolite through rat intestine from lecithin/chitosan nanoparticles. Int J Pharm 491(1–2):99–104
Feng C, Wang Z, Jiang C, Kong M, Zhou X, Li Y, Cheng X, Chen X (2013) Chitosan/o-carboxymethyl chitosan nanoparticles for efficient and safe oral anticancer drug delivery: in vitro and in vivo evaluation. Int J Pharm 457(1):158–167
Miladi K, Sfar S, Fessi H, Elaissari A (2015) Enhancement of alendronate encapsulation in chitosan nanoparticles. Journal of Drug Delivery Science and Technology 30:391–396
Diop M, Auberval N, Viciglio A, Langlois A, Bietiger W, Mura C, Peronet C, Bekel A, David DJ, Zhao M (2015) Design, characterisation, and bioefficiency of insulin–chitosan nanoparticles after stabilisation by freeze-drying or cross-linking. Int J Pharm 491(1–2):402–408
El-Shabouri M (2002) Positively charged nanoparticles for improving the oral bioavailability of cyclosporin-A. Int J Pharm 249(1–2):101–108
Samrot AV, Burman U, Philip SA, Shobana N, Chandrasekaran K (2018) Synthesis of curcumin loaded polymeric nanoparticles from crab shell derived chitosan for drug delivery. Inform Med Unlocked 10:159–182
Kaiser M, Pereira S, Pohl L, Ketelhut S, Kemper B, Gorzelanny C, Galla H-J, Moerschbacher BM, Goycoolea FM (2015) Chitosan encapsulation modulates the effect of capsaicin on the tight junctions of MDCK cells. Sci Rep 5(1):1–14
Vitale DC, Piazza C, Melilli B, Drago F, Salomone S (2013) Isoflavones: estrogenic activity, biological effect and bioavailability. Eur J Drug Metab Pharmacokinet 38(1):15–25
Richter DU, Mylonas I, Toth B, Scholz C, Briese V, Friese K, Jeschke U (2009) Effects of phytoestrogens genistein and daidzein on progesterone and estrogen (estradiol) production of human term trophoblast cells in vitro. Gynecol Endocrinol 25(1):32–38
Choi EJ, Kim GH (2014) The antioxidant activity of daidzein metabolites, O-desmethylangolensin and equol, in HepG2 cells. Mol Med Rep 9(1):328–332
Choi EJ, Kim G-H (2013) Antiproliferative activity of daidzein and genistein may be related to ERα/c-erbB-2 expression in human breast cancer cells. Mol Med Rep 7(3):781–784
Jin S, Zhang Q, Kang X, Wang J, Zhao W (2010) Daidzein induces MCF-7 breast cancer cell apoptosis via the mitochondrial pathway. Ann Oncol 21(2):263–268
Rabiau N, Kossaï M, Braud M, Chalabi N, Satih S, Bignon Y-J, Bernard-Gallon DJ (2010) Genistein and daidzein act on a panel of genes implicated in cell cycle and angiogenesis by polymerase chain reaction arrays in human prostate cancer cell lines. Cancer Epidemiol 34(2):200–206
Wu W, O’Reilly MS, Langley RR, Tsan RZ, Baker CH, Bekele N, Tang XM, Onn A, Fidler IJ, Herbst RS (2007) Expression of epidermal growth factor (EGF)/transforming growth factor-α by human lung cancer cells determines their response to EGF receptor tyrosine kinase inhibition in the lungs of mice. Mol Cancer Ther 6(10):2652–2663
Acknowledgments
The authors would like to thank the Mashhad Branch, the Islamic Azad University of Mashhad and Arka biotechnology corporation for the grant and provided chemicals and laboratory facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No potential conflict of interest was reported by the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sanatkar, R., Rahimi Kalateh Shah Mohammad, G., Karimi, E. et al. Evaluation of daidzein-loaded chitosan microcapsules for the colon cancer drug delivery: synthesis, characterization and release behaviour. Polym. Bull. 79, 7391–7405 (2022). https://doi.org/10.1007/s00289-021-03853-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-021-03853-0