Abstract
Cesium hydroxide monohydrate (CsOH·H2O) activated by cation complexing agents, i.e., 18C6 or C222 was applied as initiator of monosubstituted oxiranes polymerization. Propylene oxide (PO), 1,2-butylene oxide (BO), styrene oxide (SO) and some glycidyl ethers were used as monomers. All processes were carried out in tetrahydrofuran solution at room temperature. Such polymers, as PPO-diols, PBO-diols and PSO-diols, are unimodal and have molar masses Mn = 2000–5100. Their dispersities are rather high (Mw/Mn = 1.17–1.33). Moreover, PPO-diols and PSO-diols are not contaminated by monools with unsaturated starting groups. Poly(glycidyl ether)s are, in general, polymodal. For example, poly(isopropyl glycidyl ether)-diols are bi- or trimodal, whereas poly(allyl glycidyl ether)-diols possess two or even six fractions. Molar masses of main fraction are 4200–6400, and the second fraction is much lower, namely 600–2600. Dispersities of some fractions are very low (Mw/Mn = 1.01–1.07). Polymodality of polymers obtained was discussed in terms of the formation of two or more species propagating with different rate constants.
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polyethers are an important class of synthetic polymers, which have many applications, e.g., as impact modifiers, surfactants, de-emulsifiers, dispersant agents, fuel additives, lubricants, biomedical materials or adhesives [1,2,3,4,5]. Especially interesting are polyether- and copolyether-diols or triols prepared from oxiranes, i.e., ethylene oxide (EO), propylene oxide (PO) or 1,2-butylene oxide (BO), which are applied for fabrication of polyurethane elastomers or foams [6]. Many initiators were used for anionic ring-opening polymerization of oxiranes, e.g., potassium hydroxide [7, 8] or potassium alkoxides [9,10,11,12,13,14,15]. In industry, the most frequently used are KOH/1,2-propylene glycol or glycerol systems, which allow to obtain PPO-diols or PPO-triols with Mn = 2000–6000 at 110 °C [6, 16]. The polymers possess low unsaturation due to excess of hydroxylic compound used, which strongly limited chain transfer reaction to monomer. On the other hand, PPOs with Mn = 9000 and high unsaturation were prepared in the polymerization initiated with anhydrous KOH in THF solution at room temperature [8]. It was proposed, that in this case deprotonation of the monomer by initiator and active centers of growing chains occur to a wide extend. KOH, RbOH and CsOH are effective catalysts, while LiOH and Na OH are ineffective [17, 18]. Unsaturation of PPOs depends strongly on the nature of alkoxide cation. The relative order of alkoxide reactivity in the transfer reaction with the monomer is: Li+ > Na+ > K+ > Rb+ > Cs+ [19, 20]. This effect is rather unexpected and needs some comments. Basicity of hydroxides and alkoxides increases from Li+ to Cs+ [21]. Deprotonation of PO occurs in the complex involving monomer and active ion pair of growing chain [6, 10] (Scheme 1).
In our opinion, decreasing of deprotonation observed from Li+ to Cs+ could be explained by increasing of ionic radii of the metal cation (from 60 pm for Li+ to 169 pm for Cs+), which causes an increase in steric hindrance. On the other hand, in the polymerization initiated with potassium alkoxides and other salts activated coronand 18-crown-6 (18C6), formation of big K+18C6 complexes results in direct deprotonation of the monomer and high unsaturation [15] (Scheme 2).
It was also worth noting that PPOs synthesized in the presence of KOH/H2O/18C6 [8] or t-BuOK/t-BuOH/18C6 [14] systems have very low unsaturation and Mn due to chain transfer reaction to hydroxylic compound.
The aim of the present work was characterization of polyether-diols prepared by ring opening polymerization of several monosubstituted oxiranes initiated with cesium hydroxide monohydrate (CsOH·H2O). This reagent was useful for synthesis of dicesium 1,2-propanedioxide, which was applied for some oxiranes copolymerization [22]. However, till now, it has not been used for initiation of oxiranes polymerization. Propylene oxide, 1,2-butylene oxide, styrene oxide, isopropyl glycidyl ether, allyl glycidyl ether and phenyl glycidyl ether were chosen as monomers for the study. Polymerizations were carried out in THF solution at room temperature. The influence of initial monomer concentration and kind of ligand complexing counterion (L), i.e., coronand 18C6 or cryptand C222 on molar mass ( Mn), dispersity ( Mw/ Mn) and modality of the prepared polymers were studied and discussed. 13C NMR, MALDI-TOF and SEC techniques were used for analysis of polymers.
Experimental
Materials
Monomers, i.e., propylene oxide, 1,2-butylene oxide, styrene oxide, isopropyl glycidyl ether, allyl glycidyl ether and phenyl glycidyl ether (from Aldrich) were dried over CaH2 and distilled at 307 K (34 °C), 336 K (63 °C), 467 K (194 °C), 414 K (131 °C), 427 K (154 °C) and 518 K (245 °C), respectively. Anhydrous tetrahydrofuran (THF) (Acros Organics) was kept over CaH2 and distilled at 339 K (66 °C). Coronand 18C6 (Merck), cryptand C222 (Merck) and CsOH·H2O (Aldrich) were used without purification.
Synthesis
All syntheses were carried out at 20 °C in a 50 cm3 reactor equipped with a magnetic stirrer and a Teflon valve enabling substrates delivery and sampling under argon atmosphere. In the first series of polymerizations of propylene oxide, the initial concentration of the monomer was equal to 2.0 mol/dm3 and the initial amount of CsOH·H2O (0.34 g, 2.0 mmol) and THF (17.0 cm3) was introduced into reactor and then 18C6 (0.53 g, 2.0 mmol) was added. The mixture was stirred during 30 min. That system was used as the initiator, when the monomer (2.8 cm3, 2.4 g, 40.0 mmol) was introduced into the reactor. The reaction mixture was then stirred during 170 h. After this time complete conversion of the monomer occurred and the reaction mixture was neutralized with HCl/H2O system (0.1 mol/dm3, 50 cm3) and transferred to the separator containing chloroform (70 cm3). After shaking during 5 min, two layers were obtained, i.e., interferior polyether layer and superior layer containing water and the cesium salt. There layers were separated, and the superior layer was removed. After three washings with distilled water, polyether was obtained by evaporating of chloroform and water in vacuum at elevated temperature. In the next series, polymerizations were performed in the presence of other ligand C222 and higher initial concentration of the monomer, i.e., 5.0 mol/dm3. Similar procedure was applied for polymerization of other oxiranes. The concentration of monomer during the polymerizations was monitored by the 1,4-dioxane method [23]. The final conversions were ~ 99%. The yields of the reactions were 97−99%. All studied processes were heterogeneous and part of initiator remained at the end polymerization.
Measurements
100 MHz 13C NMR spectra were recorded in CDCl3 at 25 °C on a Bruker Avance 400 pulsed spectrometer equipped with 5 mm broad-band probe and applying Waltz16 decoupling sequence. Chemical shifts were referenced to tetramethylsilane serving as an internal standard. To obtain a good spectrum of the polymer main chain exhibiting its microstructural details, about 3000 scans were sufficient but in order to observe the signals of the polymer chain terminal groups more than 10,000 scans were necessary. Molar masses and dispersities of polymers were obtained by means of size exclusion chromatography (SEC) on a Shimadzu Prominance UFLC instrument at 40 °C on a Shodex 300 mm × 8 mm OHpac column using THF as a solvent. Polstyrenes were used as calibration standards. MALDI-TOF spectra were recorded on a Shimadzu AXIMA Performance instrument. Dithranol was used as a matrix.
Results and discussion
It was observed, that CsOH·H2O is practically insoluble in THF at room temperature and does not initiate polymerization of oxiranes. Therefore, all processes were carried out with this initiator, which is activated by macrocyclic ligands complexing metal cations, i.e., 18C6 or C222 (Scheme 3). It allowed to increase the reactions rate and yield of the polymers.
Both ligands cause ionization of initiator, which results in the formation of ligand separated ion pairs existing in equilibrium with solid salt. Thus, the concentration and reactivity of initiator increase markedly in the presence of ligand and initiator of the polymerization occurs in the solution (Scheme 4).
Due to the ratio of the ionic cesium cation radius to the 18C6 cavity radius ≈ 1.2 [24], it is theoretically possible to exist in equilibrium two kinds of complexes, i.e., flat (1/1) and sandwich (1/2) ones [25]. Cryptand C222 is able to form exclusive and inclusive complexes being in equilibrium, but in THF the formation of the inclusive complex was hindered by the strong cation–anion interaction [26].
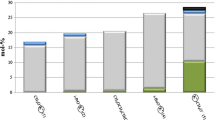
Molar masses ( Mn) of PPO-diols (1–4), PBO-diols (5–8), PSO-diols (9–12) and their dispersities ( Mw/ Mn) are collected in Table 1.
It was suggested, that initiation occurs exclusively in liquid phase, i.e., it is mediated by the part of initiator, which is soluble in the reaction mixture. It causes increase of Mn. Polarity of the medium depends on the kind and initial concentration of the monomer, which also influences Mn of polymers. Moreover, chain transfer to water results in decrease of Mn. Polymers synthesized in the presence of C222 has a little lower Mn than obtained with 18C6 at the same monomer concentration. It may result from slight increase in initiator concentration in the reaction mixture containing C222 ligand. Relatively high dispersity of the polymers obtained ( Mw/ Mn = 1.17–1.34) results from chain transfer reaction to water and slow solubilization of initiator during the polymerization. It was established, that PPO-diols and PSO-diols obtained in this work do not contain macromolecules with unsaturated starting groups. Signals in unsaturated region, i.e., at 116.7 and 134.9 ppm for PPO-diols as well at 133.2 and 159.7 ppm for PSO-diols were not observed in 13C NMR spectrum. FTIR analysis of PPOs and PSOs did not indicate signals of C=C bonds in their spectra neither at 1600–1680 (C=C bond stretching) or at 3300–3200 cm−1 (sp2 C–H bond stretching). However, such signals were detected in other systems with K+ counterion [8, 26, 27]. It indicates, that the presence of Cs+ ions and high amount of water completely eliminate unsaturation in these polymers, due to chain transfer reaction to water. In the studied systems, the rate of this reaction is evidently much more higher, than the rate of chain transfer to monomer.
Polyether-diols (1–12) are unimodal. Unexpectedly, polymers synthesized from glycidyl ethers are, in general, bimodal (13–24) (Table 2). One of them, i.e., (24) consists even six fractions.
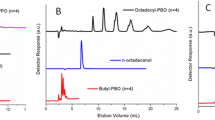
It is worth noting, that dispersities of fractions (a) are lower than (b). Dispersities of some fraction are extremely low (1.01–1.05). Figure 1 shows SEC chromatograms of two exemplary polyether-diols.
Mn of main fraction (a) of PIPGE-diol (18) obtained at [ M]o = 2.0 mol/dm3 is about two-fold higher than Mn of fraction (b). Similar effect is observed for PIPGE (20) prepared at [Mo] = 5.0 mol/dm3. Unexpectedly, Mn of fractions do not increase at much higher initial concentration of monomer. In the presence of C222, molar masses are lower. Similar phenomenon occurs in the case of PAGE-diols. The same heterogenicity and dissolving of the initiator with the course of polymerization may be responsible for the not observed increase of Mn of the product with increasing concentration of monomer (more initiator in the ionic form—smaller Mn).
MALDI-TOF spectrum of polymer (24) (Fig. 2) reveals one series of signals at m/z 954.2–6775.3. These signals represent macromolecules which consist central oxygen atom and two terminal hydroxyl groups. For example, peaks at m/z 1981.7, 3808.7 and 4379.0 belong to macromolecules possessing 17, 33 and 38 monomer units (Mcalc = 1981.4, 3809.1 and 4378.6, respectively). These macromolecules form adducts with sodium ions.
Basing on the results obtained, the course of polymerization is proposed in Scheme 5.
Formation of two or more fraction in the polymerization of some oxiranes needs some comments. It is generally accepted, that in the polymerization process bimodality exclusively appears, when there are two species, propagating with different rate constants and that these species do not exchange fast enough [28]. According to this statement, it was proposed, that in the studied systems various kinds of anionic centers with different reactivity in growing polymer chains should be formed and be responsible for polymodality of some polymers (Scheme 6).
On the other hand, the heterogenicity of the system (transfer of an initiator from solid state to liquid followed by its ionization) may be consider as a reason of polymodality. However, unimodality observed in some polyethers indicates, that formation of two or more fractions in other polymers depends also on the kind of monomer. Explanation of this phenomenon needs further investigations.
Conclusions
Cesium hydroxide monohydrate (CsOH·H2O) was applied as initiator for heterogeneous polymerization of monosubstituted oxiranes in THF at room temperature. Several systems were activated by macrocyclic ligands complexing metal cation, i.e., 18C6 or C222. Main features of prepared polyether-diols are:
-
PPO-diols, PBO-diols, PSO-diols and some PPGE-diols are unimodal; Mn of these polymers are 2000–5100, whereas dispersities are relatively high (Mw/ Mn = 1.17–1.33);
-
PPO-diols and PSO-diols are free of unsaturated monools;
-
PAGE-diols and PIPGE-diols are in general, bimodal; Mn of the fractions practically do not depend on initial monomer concentration, and their dispersities are relatively low ( Mw/Mn = 1.03–1.29);
-
Bimodality of some polymers can be explained by the formation of two species propagating with different rate constants, which do not exchange quickly enough;
-
Some of prepared polyether diols could be useful for fabrication of new polyurethane elastomers due to their relatively high Mn, low dispersity, and lack of unsaturation.
References
Zhang ZQ, Xu GY, Wang F, Dong SL, Li Y (2004) Characterization and demulsification of poly(ethylene oxide)-block-poly(propylene oxide)-block-poly(ethylene oxide) copolymers. J Colloid Interface Sci 277:464–470. https://doi.org/10.1016/j.jcis.2004.04.035
Gosa KI, Uricanu V (2002) Emulsions stabilized with PEO–PPO–PEO block copolymers and silica. Colloids Surf A 197:257–269. https://doi.org/10.1016/S0927-7757(01)00902-5
Izukawa T, Kunihiro T, (1999) Nishikawa A (Eds.), M.C.I. US Patent 5,916,994
Jeong B, Bae YH, Lee DS, Kim SW (1997) Biodegradable block copolymers as injectable drug-delivery systems. Nature 388:860–862. https://doi.org/10.1038/42218
Mathur AM, Drescher B, Scranton AB, Klier J (1998) Polymeric emulsifiers based on reversible formation of hydrophobic units. Nature 392:367–370. https://doi.org/10.1038/32856
Ionescu M (2008) Chemistry and technology of polyols for polyurethanes. Rapra Technology Limited, Shropshire
Steiner EC, Pelletier RR, Trucks RO (1964) A Study of the Polymerization of Propylene Oxide Catalyzed by Anhydrous Potassium Hydroxide. J Am Chem Soc 86:4678–4686. https://doi.org/10.1021/ja01075a031
Grobelny Z, Matlengiewicz M, Jurek J, Michalak M, Kwapulińska D, Swinarew A, Schab-Balcerzak E (2013) The influence of macrocyclic ligands and water on propylene oxide polymerization initiated with anhydrous potassium hydroxide in tetrahydrofuran. Eur Polymer J 49:3277–3288. https://doi.org/10.1016/j.eurpolymj.2013.06.035
Becker H, Wagner G (1984) Zur Übertragungsreaktion bei der anionichen Polymerisation von Oxiranen VI. Zum Einfluß von Kronenetherzusätzen auf die Polymerisation von Propylenoxid. Acta Polym 35:28–32. https://doi.org/10.1002/actp.1984.010350106
Becker H, Wagner G, Stolarzewicz A (1982) Zur Übertragungsreaktion bei der anionischen Polymerisation von Oxiranen. III. Zur Dynamik der Doppelbindungsbildung bei der Propylenoxidpolymerisation. Acta Polym 33:34–37. https://doi.org/10.1002/actp.1982.010330107
Yu GE, Heatley F, Booth C, Blease TG (1994) Anionic polymerization of propylene oxide: isomerization of allyl ether to propenyl ether end groups. J Polym Sci Part A Polym Chem 32:1131–1135. https://doi.org/10.1002/pola.1994.080320615
Ding J, Price C, Booth C (1991) Use of crown ether in the anionic polymerization of propylene oxide–1 Rate of polymerization. Eur Polym J 27:891–894. https://doi.org/10.1016/0014-3057(91)90028-M
Ding J, Heatley F, Price C, Booth C (1991) Use of crown ether in the anionic polymerization of propylene oxide−2. Molecular weight and molecular weight distribution. Eur Polymer J 27:895–899. https://doi.org/10.1016/0014-3057(91)90029-N
Grobelny Z, Swinarew A, Jurek-Suliga J, Skrzeczyna K, Gabor J, Łężniak M (2016) The influence of crown ether and alcohol on unsaturation and molar mass of poly(propylene oxide)s prepared by use of potassium t-butoxide: reinvestigation of chain transfer reactions. Int J Anal Chem. https://doi.org/10.1155/2016/3727062
Grobelny Z, Matlengiewicz M, Jurek-Suliga J, Golba S, Skrzeczyna K (2018) The influence of initiator and macrocyclic ligand on unsaturation and molar mass of poly(propylene oxide)s prepared with various anionic system. Polym Bull 75:1101–1112. https://doi.org/10.1007/s00289-017-2078-z/
Wegener G, Brandt M, Duda L, Hofmann J, Klesczewski B, Koch D, Kumpf R-J, Orzesek H, Pirkl H-G, Six Ch, Steinlein Ch, Weisbech M (2001) Trends in industrial catalysis in the polyurethane industry. Appl Catal A 221:303–335. https://doi.org/10.1016/S0926-860X(01)00910-3
PierrePrice LEStChC (1956) The room temperature polymerization of propylene oxide. J Am Chem Soc 78:3432–3436. https://doi.org/10.1021/ja01595a047
Snyder Jr W.H., Ph. D. Thesis, University of Pennsylvania, 1961
Freidly HR (1992) Reaction Polymers. In: WF Gum, W Riese, H Ulrich (Eds), Hanser Publishers: NY p. 66
Curtis CJ, Levis Jr WW, Pizzini LC inventors, BASF Wyandotte Corporation, assignee US Patent 4,487,853, 1984
Exner JH, Steiner EC (1974) Solvation and ion pairing of alkali-metal alkoxides in dimethyl sulfoxide. J Am Chem Soc 96:1782–1787. https://doi.org/10.1021/ja00813a022
Dimitrov P, Rangelov S, Dworak A, Tsvetanov ChB (2004) Synthesis and associating properties of poly(ethoxyethylglycidyl ether)/poly(propylene oxide) triblock copolymers. Macromolecules 37:1000–1008. https://doi.org/10.1021/ma0354039
Siggia S (1963) Quantitative organic analysis via functional groups. Wiley, USA, p 241
Lamb JD, Izatt RM, Christensen JJ, Eatough DJ (1979) Coordination chemistry of macrocyclic compounds. In: Melson GA (Ed.), Plenum Press, New York, p. 145–218
Issa D, Ellaboudy A, Janakiraman R, Dye JL (1984) Magnetic-susceptibilities and electron-paramagnetic resonance-spectra of a ceside and an electride. J Phys Chem 88:3847–3851. https://doi.org/10.1021/j150661a032
Kauffmann E, Dye JL, Lehn JM, Popov AI (1980) A study of the inclusive and exclusive cesium cryptates in nonaqueous solvents by cesium-133 NMR. J Am Chem Soc 102:2274–2278. https://doi.org/10.1021/ja00527a023
Grobelny Z, Matlengiewicz M, Jurek-Suliga J, Golba S, Skrzeczyna K, Kwapulińska D (2017) Ring opening polymerization of styrene oxide initiated with potassium alkoxides and hydroxyalkoxides activated by 18-crown-6: determination of mechanism and preparation of new polyether-polyols. Polym Bull 74:4763–4780. https://doi.org/10.1007/s00289-017-1976-4/
Penczek S, Cypryk M, Duda A, Kubisa P, Słomkowski S (2007) Living ring-opening polymerizations of heterocyclic monomers. Prog Polym Sci 32:247–282. https://doi.org/10.1016/j.progpolymsci.2007.01.002
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Grobelny, Z., Jurek-Suliga, J. & Golba, S. Application of cesium hydroxide monohydrate for ring opening polymerization of monosubstituted oxiranes: characterization of synthesized polyether-diols. Polym. Bull. 78, 7301–7312 (2021). https://doi.org/10.1007/s00289-020-03480-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-020-03480-1