Abstract
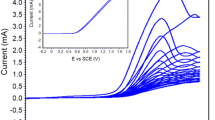
An efficient, simple, robust strategy for the protection of copper in a neutral medium has been developed by the fabrication of electropolymerised film of 2,5-dimercapto-1,3,4-thiadiazole (DMTD) + TiO2 composite. The electrosynthesised polymeric and composite films were characterised by different spectroscopic methods (FTIR, Raman spectroscopy, XPS, and XRD pattern). Electrochemical impedance spectroscopy and potentiodynamic polarisation studies confirmed the superior corrosion inhibition performance of fabricated composite film on copper in 3.5% NaCl medium. The enhanced protection efficiency of the polymeric composite could be due to the synergism between the organic polymer and inorganic particles. It is also found that the protection efficiency of the DMTD–TiO2 composite film lasts for more than 24 h. Further, SEM analysis reveals the formation of the protective film of the composite over Cu surface, while EDAX analysis unravels its composition. The study on effect of scan rate on electropolymerisation revealed that the electrodeposition of the polymer is a diffusion-controlled process. Thus, the study suggests that the fabrication of DMTD–TiO2 composite is an ideal strategy for the protection of copper in a neutral medium.
















Similar content being viewed by others
References
Tasić ŽZ, Mihajlović MBP, Radovanović MB, Antonijević MM (2019) New trends in corrosion protection of copper. Chem Pap 73(9):2103–2132
Balaji J, Sethuraman MG (2016) Corrosion protection of copper with 3-glycidoxypropyl trimethoxysilane-based sol–gel coating through 3-amino-5-mercapto-1,2,4-triazole doping. Res Chem Intermed 42:1315–1328
Recloux I, Andreatta F, Druart M, Coelho LB, Cepek C, Cossement D, Fedrizzi L, Olivier M (2018) Stability of benzotriazole-based films against AA2024 aluminium alloy corrosion process in neutral chloride electrolyte. J Alloys Compd 735:2512–2522
Wan Y, Qin Z, Xu Q, Chen M, Min YL, Li M (2017) Corrosion inhibition activity and adsorption behavior of 3-amino-1,2,4-triazole on copper. Int J Electrochem Sci 12:10701–10713
Kuznetsov YuI, Shikhaliev KhS, Agafonkina MO, Andreeva NP, Semiletov AM, Chirkunov AA, Potapov AYu, Solov’ev VE (2017) Formation of passivating layers by 1,2,4-triazole derivatives on copper in aqueous solutions. Russ J Phys Chem A 91(12):2458–2465
Farahati R, Ghaffarinejad A, Mousavi-Khoshdel SM, Rezania J, Behzadi H, Shockravi A (2019) Synthesis and potential applications of some thiazoles as corrosion inhibitor of copper in 1 M HCl: experimental and theoretical studies. Prog Org Coat 132:417–428
Pan Y, Wen Y, Guo X, Song P, Shen S, Du Y, Yang H (2013) 2-amino-5-(4-pyridinyl)-1,3,4-thiadiazole monolayers on copper surface: observation of the relationship between its corrosion inhibition and adsorption structure. Corros Sci 73:274–280
Solmaz R (2010) Investigation of the inhibition effect of 5-((E)-4-phenylbuta-1,3-dienylideneamino)-1,3,4-thiadiazole-2-thiol Schiff base on mild steel corrosion in hydrochloric acid. Corros Sci 52:3321–3330
Qin TT, Li J, Luo HQ, Li M, Li NB (2011) Corrosion inhibition of copper by 2,5-dimercapto-1,3,4-thiadiazole monolayer in acidic solution. Corros Sci 53:1072–1078
Touzé E, Cougnon C (2018) Study of the air-formed oxide layer at the copper surface and its impact on the copper corrosion in an aggressive chloride medium. Electrochim Acta 262:206–213
Fathi AM, Mandour HS (2019) Electrosynthesized conducting poly (1, 5-diaminonaphthalene) as a corrosion inhibitor for copper. Polym Bull 1:1–20
Fekri F, Zandi MS, Foroughi MM (2019) Polypyrrole coatings for corrosion protection of Al alloy2024: influence of electrodeposition methods, solvents, and ZnO nanoparticle concentrations. Iran Polym J 28(7):577–585
Sathiyanarayanan S, Devi S, Venkatachari G (2006) Corrosion protection of stainless steel by electropolymerised pani coating. Prog Org Coat 56:114–119
Krawiec H, Vignal V, Latkiewicz M, Herbst F (2018) Structure and corrosion behaviour of electrodeposited Co-Mo/TiO2 nano-composite coatings. Appl Surf Sci 427:1124–1134
Cakmakci Unver I, Bereket G, Duran B (2018) Corrosion protection of stainless steel by poly(carbazole-co-pyrrole) films deposited on TiO2 sol–gel film. Polym Plast Technol Eng 57:242–250
Chen Z, Yang W, Xu B, Guo Y, Chen Y, Yin X, Liu Y (2018) Corrosion behaviors and physical properties of polypyrrole-molybdate coating electropolymerized on carbon steel. Prog Org Coat 122:159–169
Li F, Li GX, Zeng J, Gao GH (2014) Molybdate-doped copolymer coatings for corrosion prevention of stainless steel. J Appl Polym Sci 131(16):1–8
Vinothkumar K, Sethuraman MG (2018) Corrosion inhibition ability of electropolymerised composite film of 2-amino-5-mercapto-1,3,4-thiadiazole/TiO2 deposited over the copper electrode in neutral medium. Mater Today Commun 14:27–39
Canobre SC, Biaggio SR, Rocha-Filho RC, Bocchi N (2003) Influence of the preparation procedure on the electrochemical properties of pani (DMcT-Cu ion)/carbon fiber composites. J Braz Chem Soc 14(4):621–627
Shu D, Zhang J, He C, Meng Y, Chen H, Zhang Y, Zheng M (2006) Improved electrochemical redox performance of 2,5-dimercapto-1,3,4-thiadiazole by poly(3-methoxythiophene). J Appl Electrochem 36:1427–1431
Varghese A, Chitravathi S, Munichandraiah N (2016) Electrocatalytic oxidation and determination of morin at a poly(2,5-dimercapto-1,3,4-thiadiazole) modified carbon fiber paper electrode. J Electrochem Soc 163:B471–B477
Picart S, Genies E (1996) Electrochemical study of 2,5-dimercapto-1,3,4-thiadiazole in acetonitrile. J Electroanal Chem 408:53–60
Elbakri M, Touir R, Ebn Touhami M, Srhiri A, Benmessaoud M (2008) Electrosynthesis of adherent poly(3-amino-1,2,4-triazole) films on brass prepared in nonaqueous solvents. Corros Sci 50:1538–1545
Joy VT, Srinivasan TKK (2001) FT-SERS studies on 1,3-thiazolidine-2-thione, 2,5-dimercapto-1,3,4-thiadiazole and 2-thiouracil adsorbed on chemically deposited silver films. J Raman Spectrosc 32:785–793
Chen W, Luo HQ, Li NB (2011) Inhibition effects of 2,5-dimercapto-1,3,4-thiadiazole on the corrosion of mild steel in sulphuric acid solution. Corros Sci 53:3356–3365
Huang L, Tang F, Hu B, Shen J, Yu T, Meng Q (2001) Chemical reactions of 2,5-dimercapto-1,3,4-thiadiazole (DMTD) with metallic copper, silver, and mercury. J Phys Chem B 105:7984–7989
Thamaphat K, Limsuwan P, Ngotawornchai B (2008) Phase characterization of TiO2 powder by XRD and TEM. Kasetsart J Nat Sci 42(5):357–361
Zhang J, Boyd IW, Sullivan BJO, Hurley PK, Kelly PV (2002) Nanocrystalline TiO2 films studied by optical, XRD and FT-IR spectroscopy. J Non-Cryst Solids 303:134–138
Brust M, Blass PM, Bard AJ (1997) Self-assembly of photoluminescent copper (I)—dithiol multilayer thin films and bulk materials. Langmuir 13:5602–5607
Yang J, Wang Y, Li W, Wang L, Fan Y, Jiang W, Luo W, Wang Y, Kong B, Selomulya C, Liu HK, Dou SX, Zhao D (2017) Amorphous TiO2 Shells: A vital elastic buffering layer on silicon nanoparticles for high-performance and safe lithium storage. Adv Mater 29:1700523(1)–1700523(7)
Lewin E, Gorgoi M, Schäfers F, Svensson S, Jansson U (2009) Influence of sputter damage on the XPS analysis of metastable nanocomposite coatings. Surf Coat Technol 204:455–462
Merel P, Tabbal M, Chaker M, Moisa S, Margot J (1998) Direct evaluation of the sp3 content in diamond-like-carbon films by XPS. Appl Surf Sci 136:105–110
Lisowska-Oleksiaka A, Nowaka NP, Wilamowskaa M, Sikorab M, Szczerbab W, Kapustab C (2010) Ex situ XANES, XPS and Raman studies of poly(3,4-ethylenedioxythiophene) modified by iron hexacyanoferrate. Synth Met 160:1234–1240
Rajkumar G, Sethuraman MG (2016) A novel hybrid composite coating of poly-3-amino-5-mercapto-1,2,4-triazole/TiO2 on copper for corrosion protection. Iran Polym J 25:119–128
Balaji J, Sethuraman MG (2016) Improved corrosion resistance by forming multilayers over a copper surface by electrodeposition followed by a novel sol–gel coating method. RSC Adv 6:95396–95404
Wu X (1999) General equivalent circuits for faradaic electrode processes under electrochemical reaction control. J Electrochem Soc 146:1847–1853
Babhu Vignesh R, Sethuraman MG (2014) Enhancement of corrosion protection of 3-glycidoxyproyltrimethoxysilane-based sol-gel coating through methylthiourea doping. J Coat Technol Res 11:545–554
Chen W, Hong S, Luo HQ, Li NB (2014) Inhibition effect of 2,4,6-trimercapto-1,3,5-triazine self-assembled monolayers on copper corrosion in NaCl solution. J Mater Eng Perform 23:527–537
Zarrouk A, Hammouti B, Dafali A, Bentiss F (2013) Inhibitive properties and adsorption of purpald as a corrosion inhibitor for copper in nitric acid medium. Ind Eng Chem Res 52:2560–2568
Rajkumar G, Sethuraman MG (2013) Electrosynthesis of a novel poly(3-amino-1,2,4-triazole) + TiO2 hybrid composite on copper and its corrosion protection. Ind Eng Chem Res 52(43):15057–15065
Acknowledgements
The authors would like to thank the CSIR EMR-II Division for the financial support through the major research project (Ref. No: 01(2842)/16/EMR-II dated 12/05/2016). Also the authors would like to thank the authorities of the Gandhigram Rural Institute (Deemed to be University), Gandhigram, for their support and encouragement.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vinothkumar, K., Sethuraman, M.G. Protection of copper from corrosion through electrodeposited poly-2,5-dimercapto-1,3,4-thiadiazole–TiO2 composite film. Polym. Bull. 78, 15–34 (2021). https://doi.org/10.1007/s00289-019-03090-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-019-03090-6