Abstract
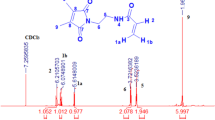
Vanillin acrylate (VA) monomer was prepared and elucidated by 1H NMR 13C and FTIR. Three different mole ratios of smart functional photo-cross-linker polymers were synthesized by the copolymerization of N-isopropylacrylamide (5, 10 and 15 mol%) VA functional monomer and (2, 5 and 10 mol%) photo-cross-linkers. On the other hand, three different mole ratios (2, 5 and 10 mol%) of thermo-responsive photo-cross-linker polymers were prepared by copolymerization of (NIPAAm) and (DIMAAm). Polymers were characterized by 1HNMR, FTIR, UV, gel permeation chromatography (GPC) and differential scanning calorimetery (DSC). Lower critical solution temperatures (Tc) were determined by UV–Vis. Spectroscopy and micro-DSC. Hydrogel bilayer was formed by spin coating of polymer solution of poly(N-isopropylacrylamide-co-maleimide) layer A over gold with adhesion promoter, and then cross-linked by UV-irradiation. The next layer was formed by spin coating of polymer solution poly(N-isopropylacryamide-co-malimide-co-VA) layer B over layer A, and then cross-linked by UV-irradiation. The swelling properties and Tc were determined by SPR/OW. Our target is the formation of active centre for biological molecules using aldehyde group, which act as biosensor gel vessel. Further, the hydrogel bilayer will facilitate the target molecule to stay safely inside this gel vessel responsive to temperature and pH.
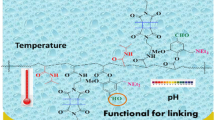
Graphical abstract
New acrylate monomer from vanillin has been synthesized with aldehyde group to achieve both functionality and pH responsive. The copolymerization with NIPAAm and DMIAAm has done forming pH and temperature photo-cross-linked polymers. Hydrogel layer was built first with single then bilayer and both has characterized by SPR/OW. The effect of Temperature-responsive layer on the dual responsive one was demonstrated through the change lower critical solution temperature. In the nearest future, we will use this technique as biosensor hydrogel for biotechnology e.g., drug delivery and bio-separation.




















Similar content being viewed by others
References
Deshmukh PK, Ramani KP, Singh SS, Tekade AR, Chatap VK, Patil GB, Bari SB (2013) Stimuli-sensitive layer-by-layer (LbL) self-assembly systems: targeting and biosensory applications. J Control Release 166:294–306. https://doi.org/10.1016/j.jconrel.2012.12.033
Kikuchi A, Okano T (2002) Polymerization of oligo(ethylene glycol) (meth)acrylates: toward new generations of smart biocompatible materials. Prog Polym Sci 27:1165–1175. https://doi.org/10.1002/pola.22706
Ware T, Simon D, Rennaker RL, Voit W (2013) Thiol-click chemistries for responsive neural interfaces smart polymers for neural interfaces. Polym Rev 53(108):1640–1647. https://doi.org/10.1002/mabi.201300272
Hamner KL, Alexander CM, Coopersmith K, Reishofer D, Provenza C, Maye MM (2013) Using temperature-sensitive smart polymers to regulate DNA-mediated nanoassembly and encoded nanocarrier drug release. ACS Nano 7:7011–7020. https://doi.org/10.1021/nn402214e
Qiu Y, Park K (2001) Environment-sensitive hydrogels for drug delivery. Adv Drug Deliv Rev 53:321
Sato E, Masuda Y, Kadota J, Nishiyama T, Horibe H (2015) Dual stimuli-responsive homopolymers: thermo- and photo-responsive properties of coumarin-containing polymers in organic solvents. Eur Polym J 69:605–615
Abdelaty MSA (2017) Environmental functional photo-cross-linked hydrogel bilayer thin films from vanillin. J Polym Environ 3:1–14. https://doi.org/10.1007/s10924-017-1126-y
Abdelaty MSA (2017) Preparation and characterization of new environmental functional polymers based on vanillin and N isopropylacrylamide for post polymerization. J Polym Environ 3:1–11. https://doi.org/10.1007/s10924-017-0960-2
Abdelaty MSA, Kuckling D (2016) Synthesis and characterization of new functional photo-cross-linkable smart polymers containing vanillin derivatives. Gels 2:1–13. https://doi.org/10.1016/j.eurpolymj.2015.05.010
Meng H, Mohamadian H, Stubblefield M, Jerro D, Ibekwe S, Pang SS, Li GQ (2013) Fabrications and applications of stimulus-responsive polymer films and patterns on surfaces: a review. Smart Mater Struct 22:805–875. https://doi.org/10.3390/ma7020805
Zhang M, Estournes C, Bietsch W, Mueller AHE (2004) Superparamagnetic hybrid nanocylinders. Adv Funct Mater 14:871–882
Matsukuma D, Yamamoto K, Aoyagi T (2006) Stimuli-responsive properties of N-isopropylacrylamide-based ultrathin hydrogel films prepared by photo-cross-linking. Langmuir 22:5911–5915. https://doi.org/10.1021/la060438y
Chen Y, Pang XH, Dong CM (2010) Dual stimuli-responsive supramolecular polypeptide-based hydrogel and reverse micellar hydrogel mediated by host-guest chemistry. Adv Funct Mater 20:579–586. https://doi.org/10.1002/adfm.200901400
Schattling P, Jochum F-D, Theato P (2014) Multi-stimuli responsive polymers—the all-in-one talents. Poly Chem 5:25–36. https://doi.org/10.1039/C3PY00880K
Li Y, Zhang C, Zhou Y, Dong Y, Chen W (2015) Novel multi-responsive polymer materials: when ionic liquids step in. Eur Polym J 69:441–448. https://doi.org/10.1016/j.eurpolymj.2015.05.023
Fujiwara N, Asaka K, Nishimura Y, Oguro K, Torikai E (2000) Preparation of gold–solid polymer electrolyte composites as electric stimuli-responsive materials. Chem Mater 12:1750–1754. https://doi.org/10.1021/cm9907357
Roy D, Cambre JN, Sumerlin BS (2011) In: Urban MW (eds) Handbook of stimuli-responsive materials. Wiley, Weinheim, pp 122–125
Xia Y, Yin X, Burke NAD, Stoever HDH (2005) Thermal response of narrow-disperse poly (N-isopropylacrylamide) prepared by atom transfer radical polymerization. Macromolecules 38:5937–5943. https://doi.org/10.1021/ma050261z
Cheng G, Boeker A, Zhang M, Krausch G, Mueller AHE (2001) Amphiphilic cylindrical core–shell brushes via a “Grafting From” process using ATRP. Macromolecules 34:6883–6892. https://doi.org/10.1021/ma0013962
Chen H, Shao L, Choi K, Wang J, Lin HQ (2012) Plasmonic-molecular resonance coupling: plasmonic splitting versus energy transfer. J Phys Chem C 116:14088–14095. https://doi.org/10.1021/jp303560sJ
Seuring S Agarwal (2012) Polymers with upper critical solution temperature in aqueous solution. Macromol Rapid Commun 33:1898–1920. https://doi.org/10.1002/marc.201200433
Chang K, Rubright N, Lowery PD, Taite LJ (2013) Structural optimization of highly branched thermally responsive polymers as a means of controlling transition temperature. J Polym Sci A Polym Chem 51:2068–2078. https://doi.org/10.1002/pola.26596
Heskins M, Guillet JE (1968) Solution properties of poly(N-isopropylacrylamide). J Macromol Sci Chem A 2:1441–1455. https://doi.org/10.1080/10601326808051910
Okubo M, Ahmad H, Suzuki T (1998) Synthesis of temperature-sensitive micron-sized monodispersed composite polymer particles and its application as a carrier for biomolecules. Colloid Polym Sci 276:470. https://doi.org/10.1007/s003960050268
Iatridi Z, Mattheolabakis G, Avgoustakis K, Tsitsilianis C (2011) Self-assembly and drug delivery studies of pH/thermo-sensitive polyampholytic (A-co-B)-b-C-b-(A-co-B) segmented terpolymers. Soft Mater 7:11160–11168. https://doi.org/10.1039/C1SM06185B
Soppimath KS, Tan DCW, Yang YY (2005) pH-Triggered thermally responsive polymer core-shell nanoparticles for drug delivery. Adv Mater 17:318–323. https://doi.org/10.1002/adma.200401057
Delcea M, Möhwald H, Skirtach AG (2011) Stimuli-responsive LbL capsules and nanoshells for drug delivery. Adv Drug Deliv Rev 63:730–747. https://doi.org/10.1016/j.addr.2011.03.010
Uhlig K, Boysen B, Lankenau A, Jaeger M, Wischerhoff E, Lutz JF, Laschewsky A, Duschl C (2012) On the influence of the architecture of poly(ethylene glycol)-based thermoresponsive polymers on cell adhesion. Biomicrofluidics 6:11–21. https://doi.org/10.1063/1.4729130
Skirtach AG, Yashchenok AM, Möhwalda H (2011) Encapsulation, release and applications of LbL polyelectrolyte multilayer capsules. Chem Commun 47:12736–12746. https://doi.org/10.1039/C1CC13453A
Bedard M, Skirtach AG, Sukhorukov GB (2007) Optically driven encapsulation using novel polymeric hollow shells containing an azobenzene polymer. Macromol Rapid Commun 28:1517–1521. https://doi.org/10.1002/marc.200700257
Honda M, Kataoka K, Seki T, Takeoka Y (2009) Confined stimuli-responsive polymer gel in inverse opal polymer membrane for colorimetric glucose sensor. Langmuir 25:8349–8356. https://doi.org/10.1021/la804262b
Fleischmann EK, Zentel R (2013) Liquid-crystalline ordering as a concept in materials science: from semiconductors to stimuli-responsive devices. Angew Chem Int Ed 52:8810–8827. https://doi.org/10.1002/anie.201300371
Zhang C, Madbouly SA, Kessler MR (2015) Soybean-oil-based thermosetting resins with methacrylated vanillyl alcohol as bio-based, low-viscosity comonomer. Macromol Chem Phys 216:1816–1826. https://doi.org/10.1002/mame.201700278
Stanzione JF, Sadler JM, La Scala JJ, Wool RP (2012) Lignin model compounds as bio-based reactive diluents for liquid molding resins. Chem Sustain Chem 5:1291–1297. https://doi.org/10.1002/cssc.201100687
Fache M, Darroman E, Besse V, Auvergne R, Sylvain S, Caillol B (2014) Vanillin, a promising biobased building-block for monomer synthesis. Boutevina Green Chem 16:1987–1998. https://doi.org/10.1039/C3GC42613K
Mateescu A, Wang Y, Dostalek J, Jonas U (2012) Thin hydrogel films for optical biosensor applications. Membranes 2:40–69. https://doi.org/10.3390/membranes2010040
Feng X, Wu H, Sui X, Hempenius MA, JuliusVancso G (2015) Vancso, Thin film hydrogels from redox responsive poly(ferrocenylsilanes): preparation, properties, and applications in electrocatalysis. Eur Polym J 72:535–542. https://doi.org/10.1016/j.eurpolymj.2015.05.022
Tokarev I, Minko S (2009) Stimuli-responsive hydrogel thin films. Soft Mater 5:511–524. https://doi.org/10.1039/B813827C
Harmon ME, Kuckling D, Pareek P, Frank CW (2003) Photo-cross-linkable PNIPAAm copolymers. 5. Mechanical properties of hydrogel layers. Langmuir 19:10660–10665. https://doi.org/10.1021/la030232m
Kuckling D, Harmon ME, Frank CW (2002) A surface plasmon resonance study of volume phase transitions in N-isopropylacrylamide gel films. Macromolecules 35:5999–6004. https://doi.org/10.1021/ma010985k
Harmon ME, Kuckling D, Frank CW (2003) Photo-cross-likable PNIPAAm copolymer 2: effects of constraint on temperature pH responsive hydrogel layers. Macromolecules 36:162–172. https://doi.org/10.1021/ma021025gCCC
Nan Zhang N, Knoll W (2009) Thermally responsive hydrogel films studied by surface plasmon diffraction. Anal Chem 81:2611–2617I. https://doi.org/10.1021/ac802527j
Aulasevich A, Junk MJN, Jakubowicz P, Roskamp RF, Menges B, Jonas U, Knoll W (2010) SPR/OWS angle spectra of (A) p-polarized and (B) s-polarized ligh. Macrmol Chem Phys 211:1018–1025. https://doi.org/10.1002/macp.200900533
Chen JK, Chan CH, Chang FC (2008) Phase change memory cell using tungsten trioxide bottom heating layer. Appl Phys Lett 92(053108):1. https://doi.org/10.1063/1.2939218
Chan CH, Chen JK, Chang FC (2008) Specific DNA extraction through fluid channels with immobilization of layered double hydroxides on polycarbonate surface. Sens Actuat B 133:327–332. https://doi.org/10.1016/j.snb.2008.02.041
Chen JK, Li JY (2010) Fabrication of DNA extraction device with tethered poly(N-isopropylacrylamide) brushes on silicon surface for a specific DNA detection. Sens Actuat B 150:314–320. https://doi.org/10.1016/j.snb.2010.06.067
Hosoya K, Kubo T, Tanaka N, Haginaka JA (2003) A possible purification method of DNAs’ fragments from humic matters in soil extracts using novel stimulus responsive polymer adsorbent. J Pharm Biomed Anal 30:1919–1922
Lu Y, Mei Y, Drechsler M, Ballauff M (2006) Thermosensitive core–shell particles as carriers for Ag nanoparticles: modulating the catalytic activity by a phase transition in networks. Angew Chem Int Ed 45:813–816. https://doi.org/10.1002/anie.200502731
Chen JK, Wang JH, Chang JY, Fan SK (2012) Thermally switchable adhesions of polystyrene-block-poly(n-isopropylacrylamide) copolymer pillar array mimicking climb attitude of geckos. Appl Phys Lett 101(123701):1. https://doi.org/10.1063/1.4754135
Costa E, Coelho M, Ilharco LM, Aguiar-Ricardo A, Hammond PT (2011) Tannic acid mediated suppression of PNIPAAm microgels thermoresponsive behavior. Macromolecules 44:612–621. https://doi.org/10.1021/ma1025016
Yang HW, Chena JK, Cheng CC, Kuo SW (2013) Association of poly(N-isopropylacrylamide) containing nucleobase multiple hydrogen bonding of adenine for DNA recognition. Appl Surf Sci 271:60–69. https://doi.org/10.1016/j.apsusc.2013.01.074
Gauthier MA, Gibson MI, Klok HA (2009) Synthesis of functional polymers by post-polymerization modification. Angew Chem Int Ed 48:48–58. https://doi.org/10.1002/anie.200801951
Fuchs AD, Tiller JC (2006) Contact-active antimicrobial coatings derived from aqueous suspensions. Angew Chem Int Ed 45:6759–6762. https://doi.org/10.1002/anie.200602738
Zolotukhin MG, Colquhoun HM, Sestiaa LG, Rueda DR, Flot D (2003) One-pot synthesis and characterization of soluble poly(aryl ether-ketone)s having pendant carboxyl groups. Macromolecule 36:4766–4771. https://doi.org/10.1021/ma0216977
Brooks YE, Kizhakkedathu JN (2008) A novel functional polymer with tunable LCST. Macromolecules 41:5393–5405. https://doi.org/10.1021/ma8006703
Schneider BH, Dickinson EL, Vach MD, Hoijer JV, Howard LV (2000) Highly sensitive optical chip immunoassays in human serum. Biosens Bioelectron 15:13–22. https://doi.org/10.1016/S0956-5663(00)00056-7
Vaisocherova H, Yang W, Zhang Z, Cao ZQ, Cheng G, Piliarik M, Homola J, Jiang SY (2008) Ultralow fouling and functionalizable surface chemistry based on a zwitterionic polymer enabling sensitive and specific protein detection in undiluted blood plasma. Anal Chem 80:7894–7901. https://doi.org/10.1021/ac8015888
Wark AW, Lee HJ, Corn RM (2005) Long-range surface plasmon resonance imaging for bioaffinity sensors. Anal Chem 77:3904–3907. https://doi.org/10.1021/ac050402v
Lakhiari H, Okano T, Nurdin N, Luthi DP, Muller D, Jozefonvicz J (1998) Temperature-responsive size-exclusion chromatography using poly(N-isopropylacrylamide) grafted silica. Biochimica Et Biophysica Acta-General Subjects 1379:303–313. https://doi.org/10.1016/S0304-4165(97),00110-4
Kanazawa H, Yamamoto K, Matsushima Y, Takai N, Kikuchi A, Sakurai Y, Okano T (1996) Temperature-responsive chromatography using poly(N-isopropylacrylamide)-modified silic. Anal Chem 68:100–105. https://doi.org/10.1021/ac950359j
Bhattacharya S, Eckert F, Boyko V, Pich A (2007) Temperature-, pH-, and magnetic-field-sensitive hybrid microgels. Small 3:650–657. https://doi.org/10.1002/smll.200600590
Xulu PM, Filipcsei G, Zrinyi M (2000) Preparation and responsive properties of magnetically soft poly(N-isopropylacrylamide) gels. Macromolecules 33:1716–1719. https://doi.org/10.1021/ma990967r
Acknowledgements
The authors acknowledge to Egyptian culture and missions, and The Deutscher Akademischer Austauch (DAAD) for financial assistance during the post-doctor work in Germany of Momen S.A. Abdelaty.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares no conflict of interest.
Rights and permissions
About this article
Cite this article
Abdelaty, M.S.A. Environmental functional photo-cross-linked hydrogel bilayer thin films from vanillin (part 2): temperature-responsive layer A, functional, temperature and pH layer B. Polym. Bull. 75, 4837–4858 (2018). https://doi.org/10.1007/s00289-018-2297-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-018-2297-y