Abstract
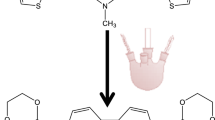
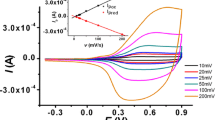
In the present work, the synthesis and study of the electrochemical behavior of 2,7-bis(3-octylthiophene-2-yl)-N-methylcarbazole are reported. The electropolymerization of this monomer was achieved by potentiodynamic cyclic voltammetry and potentiostatic techniques. Voltammograms show that two different conducting deposits are formed on Pt for oxidation at 1.0 and 0.8 V. The nature of the deposits depends on the applied potential and the scan rate. Both deposits exhibit n- and p-doping/undoping processes that are thermodynamically reversible and kinetically partially reversible. The same behavior was determined on fluorine-doped tin oxide. The nucleation and growth mechanism (NGM) of the electropolymerization were investigated using a potentiostatic technique (i–t). Two mechanisms were obtained, and the deconvolution of the current–time transient for the low-potential data fitted with a theoretical model suggesting that at short times, an instantaneous nucleation with a two-dimensional growth contribution prevails, followed by a three-dimensional progressive nucleation with a transfer charge diffusion. Finally, at longer times, a three-dimensional progressive nucleation with a diffusion-controlled growth contribution becomes important. The morphology predicted from these NGMs fully correlates with that determined by scanning electron microscopy.








Similar content being viewed by others
References
Bian L, Zhu E, Tang J, Tang W, Zhang F (2012) Recent progress in the design of narrow bandgap conjugated polymers for high-efficiency organic solar cells. Prog Polym Sci 37:1292–1331
Nambiar S, Yeow JTW (2011) Conductive polymer-based sensors for biomedical applications. Biosens Bioelectron 26:1825–1832
Guo X, Baumgarten M, Müllen K (2013) Designing π-conjugated polymers for organic electronics. Prog Polym Sci 38:1832–1908
Argun AA, Aubert PH, Thompson BC, Schwendeman I, Gaupp CL, Hwang J, Pinto NJ, Tanner DB, MacDiarmid AG, Reynolds JR (2004) Multicolored electrochromism in polymers: structures and devices. Chem Mater 16:4401–4412
Cheng Y-J, Yang S-H, Hsu C-S (2009) Synthesis of conjugated polymers for organic solar cell applications. Chem Rev 109:5868–5923
Singh TB, Sariciftci NS (2006) Progress in plastic electronics devices. Annu Rev Mater Res 36:199–230
Fichou D (1999) Handbook of Oligo- and Polythiophene. Wiley-VCH Verlag GmbH & Co, KGaA
Osaka I, Mccullough RD (2008) Advances in molecular design and synthesis of regioregular polythiophenes. Acc Chem Res 41:1202–1214
Bhadra S, Khastgir D, Singha NK, Lee JH (2009) Progress in preparation, processing and applications of polyaniline. Prog Polym Sci 34:783–810
Jaymand M (2013) Recent progress in chemical modification of polyaniline. Prog Polym Sci 38:1287–1306
Zhang S, Wang G, Lv G, Yu D, Ding Y (2014) Synthesis and fluorescence properties of a soluble polypyrrole derivative based on a dipyrrole monomer. Synth Met 195:185–192
Chougule MA (2011) Synthesis and characterization of polypyrrole (PPy) thin films. Soft Nanosci Lett 01:6–10
Neher D (2001) Polyfluorene homopolymers: conjugated liquid-crystalline polymers for bright blue emission and polarized electroluminescence. Macromol Rapid Commun 22:1365–1385
Kitazawa D, Watanabe N, Yamamoto S, Tsukamoto J (2012) Conjugated polymers based on quinoxaline for polymer solar cells. Sol Energy Mater Sol Cells 98:203–207
Leclerc M (2001) Polyfluorenes: twenty years of progress. J Polym Sci Part A: Polym Chem 39:2867–2873
Morin J-F, Leclerc M, Adès D, Siove A (2005) Polycarbazoles: 25 years of progress. Macromol Rapid Commun 26:761–778
Zou Y, Gendron D, Badrou-Aïch R, Najari A, Tao Y, Leclerc M (2009) A high-mobility low-bandgap poly(2,7-carbazole) derivative for photovoltaic applications. Macromolecules 42:2891–2894
Blouin N, Leclerc M (2008) Poly(2,7-carbazole)s: structure—property Relationships. Acc Chem Res 41:110–119
Yi H, Al-Faifi S, Iraqi A, Watters DC, Kingsley J, Lidzey DG (2011) Carbazole and thienyl benzo[1,2,5]thiadiazole based polymers with improved open circuit voltages and processability for application in solar cells. J Mater Chem 21:13649
Lee SK, Lee WH, Cho JM, Park SJ, Park JU, Shin WS, Lee JC, Kang IN, Moon SJ (2011) Synthesis and photovoltaic properties of quinoxaline-based alternating copolymers for high-efficiency bulk-heterojunction polymer solar cells. Macromolecules 44:5994–6001
Lu J, Liang F, Drolet N, Ding J, Tao Y, Movileanu R (2008) Crystalline low band-gap alternating indolocarbazole and benzothiadiazole-cored oligothiophene copolymer for organic solar cell applications. Chem Commun 5315–5317. doi:10.1039/b811031j
Blouin N, Michaud A, Leclerc M (2007) A low-bandgap poly(2,7-carbazole) derivative for use in high-performance solar cells. Adv Mater 19:2295–2300
Boudreault P-LT, Beaupré S, Leclerc M (2010) Polycarbazoles for plastic electronics. Polym Chem 1:127–136
Pan J, Chiu H, Wang B (2005) Theoretical investigation of carbazole derivatives as hole-transporting materials in OLEDs. J Mol Struct (Theochem) 725:89–95
Tran-Van F, Henri T, Chevrot C (2002) Synthesis and electrochemical properties of mixed ionic and electronic modified polycarbazole. Electrochim Acta 47:2927–2936
Li Y, Wu Y, Ong BS (2006) Polyindolo[3,2-b]carbazoles: a new class of p-channel semiconductor polymers for organic thin-film transistors. Macromolecules 39:6521–6527
Roncali J (1992) Conjugated Poiy(thiophenes): synthesis, functionalization, and applications. Chem Rev 92:711–738
Grazulevicius JV, Strohriegl P, Pielichowski J, Pielichowski K (2003) Carbazole-containing polymers: synthesis, properties and applications. Prog Polym Sci 28:1297–1353
Heinze J, Frontana-Uribe BA, Ludwigs S (2010) Electrochemistry of conducting polymers–persistent models and new concepts. Chem Rev 110:4724–4771
Li C, Bai H, Shi G (2009) Conducting polymer nanomaterials: electrosynthesis and applications. Chem Soc Rev 38:2397–2409
Xia X, Chao D, Qi X, Xiong Q, Zhang Y, Tu J, Zhang H, Fan HJ (2013) Controllable growth of conducting polymers shell for constructing high-quality organic/inorganic core/shell nanostructures and their optical-electrochemical properties. Nano Lett 13:4562–4568
del Valle M, Cury P, Schrebler R (2002) Solvent effect on the nucleation and growth mechanisms of poly(thiophene). Electrochim Acta 48:397–405
Arteaga GC, Valle MA, Antilén M, Romero M, Ramos A, Hernández L, Arévalo MC, Pastor E, Louarn G (2013) Nucleation and Growth Mechanism of Electro-synthesized Poly (pyrrole) on Steel. Int J Electrochem Sci 8:4120–4130
Ugalde L, Bernede JC, Del Valle MA, Diaz FR, LeRay P (2002) SEM study of the growth of electrochemically obtained polythiophene thin films: effect of electrolyte and monomer concentration in acetonitrile. J Appl Polym Sci 84:1799–1809
Aristizabal JA, Soto JP, Ballesteros L, Muñoz E, Ahumada JC (2012) Synthesis, electropolymerization, and photoelectrochemical characterization of 2,7-di(thiophen-2-yl)-N-methylcarbazole. Polym Bull 70:35–46
Pletcher JRD, Greff R, Peat R, Peter LM (2011) Instrumental methods in electrochemistry. Woodhead Publishing Limited, Cambridge
Baycan Koyuncu F, Koyuncu S, Ozdemir E (2011) A new multi-electrochromic 2,7 linked polycarbazole derivative: effect of the nitro subunit. Org Electron Phys Mater Appl 12:1701–1710
Abdulla HS, Spectroscopy I (2013) Electrochemical synthesis and vibrational mode analysis of poly (3-methylthiophene). Int J Electrochem Sci 8:11782–11790
Sonone R, Raut V, Murhekar G (2014) Structural and electroluminescence properties of pure PVK and doped Tio 2 polymer thin films. Int J Adv Res Chem Sci 1:87–94
Acknowledgments
The authors acknowledge the financial support from the FONDECYT Project No. 11080073 and the DII-Pontificia Universidad Católica de Valparaíso and are also grateful to CONICYT-FONDEQUIP program NMR 300 (Grant Number EQM 130154) and CONICYT-Beca Doctorado Nacional number 21120405.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aristizabal, J.A., Ahumada, J.C. & Soto, J.P. Electrochemical preparation and characterization of a new conducting copolymer of 2,7-carbazole and 3-octylthiophene. Polym. Bull. 74, 1649–1660 (2017). https://doi.org/10.1007/s00289-016-1794-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-016-1794-0