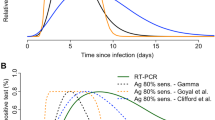
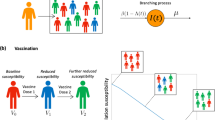
Abstract
Travel restrictions, while delaying the spread of an emerging disease from the source, could inflict substantial socioeconomic burden. Travel-related policies, such as quarantine and testing of travelers, may be considered as alternative strategies to mitigate the negative impact of travel bans. We developed a meta-population, delay-differential model to evaluate a strategy that combines testing of travelers prior to departure from the source of infection with quarantine and testing at exit from quarantine in the destination population. Our results, based on early parameter estimates of SARS-CoV-2 infection, indicate that testing travelers at exit from quarantine is more effective in delaying case importation than testing them before departure or upon arrival. We show that a 1-day quarantine with an exit test could outperform a longer, 3-day quarantine without testing in delaying the outbreak peak. Rapid, large-scale testing capacities with short turnaround times provide important means of detecting infectious cases and reducing case importation, while shortening quarantine duration for travelers at destination.




Similar content being viewed by others
References
Alexander ME, Moghadas SM, Röst G, Wu J (2008) A delay differential model for pandemic influenza with antiviral treatment. Bull Math Biol 70(2):382–397
Alfano V, Ercolano S (2020) The efficacy of lockdown against COVID-19: a cross-country panel analysis. Appl Health Econ Health Policy 18:509–517
Arino J, Van Den Driessche P (2006) Time delays in epidemic models: modeling and numerical considerations. In: Delay differential equations and applications. Springer, pp 539–578
Bou-Karroum L, Khabsa J, Jabbour M, Hilal N, Haidar Z, Abi Khalil P, Khalek RA, Assaf J, Honein-AbouHaidar G, Abou Samra C et al (2021) Public health effects of travel-related policies on the COVID-19 pandemic: a mixed-methods systematic review. J Infect 83(4):413–423
Brownstein JS, Wolfe CJ, Mandl KD (2006) Empirical evidence for the effect of airline travel on inter-regional influenza spread in the United States. PLoS Med 3(10):401
Chinazzi M, Davis JT, Ajelli M, Gioannini C, Litvinova M, Merler S, Pastore y Piontti A, Mu K, Rossi L, Sun K et al (2020) The effect of travel restrictions on the spread of the 2019 novel coronavirus (COVID-19) outbreak. Science 368(6489):395–400
Di Domenico L, Pullano G, Sabbatini CE, Boëlle P-Y, Colizza V (2020) Impact of lockdown on COVID-19 epidemic in Île-de-France and possible exit strategies. BMC Med. https://doi.org/10.1186/s12916-020-01698-4
Dickens BL, Koo JR, Lim JT, Sun H, Clapham HE, Wilder-Smith A, Cook AR (2020) Strategies at points of entry to reduce importation risk of COVID-19 cases and reopen travel. J Travel Med 27(8):141
Diekmann O, Heesterbeek JAP (2000) Mathematical epidemiology of infectious diseases: model building, analysis and interpretation, vol 5. Wiley, New York
Findlater A, Bogoch II (2018) Human mobility and the global spread of infectious diseases: a focus on air travel. Trends Parasitol 34(9):772–783
Germann TC, Kadau K, Longini IM, Macken CA (2006) Mitigation strategies for pandemic influenza in the United States. Proc Natl Acad Sci 103(15):5935–5940
Hollingsworth TD, Ferguson NM, Anderson RM (2006) Will travel restrictions control the international spread of pandemic influenza? Nat Med 12(5):497–499
International Air Transport Association (IATA) (2020) Air connectivity: measuring the connections that drive economic growth. Technical report
Klinger C, Burns J, Movsisyan A, Biallas R, Norris SL, Rabe JE, Stratil JM, Voss S, Wabnitz K, Rehfuess EA et al (2021) Unintended health and societal consequences of international travel measures during the COVID-19 pandemic: a scoping review. J Travel Med 28(7):123
Lee FW, Wang J, Wang CJ (2022) A testing and quarantine algorithm for individual international travelers using published data on WHO-approved vaccines and Bayes’ Theorem. Vaccines 10(6):902
Leung K, Wu JT, Leung GM (2021) Effects of adjusting public health, travel, and social measures during the roll-out of COVID-19 vaccination: a modelling study. Lancet Public Health 6(9):674–682
Li R, Pei S, Chen B, Song L, Zhang T, Yang W, Shaman J (2020) Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus. Science 368(6490):489–493
Moghadas SM, Fitzpatrick MC, Sah P, Pandey A, Shoukat A, Singer BH, Galvani AP (2020) The implications of silent transmission for the control of COVID-19 outbreaks. Proc Nal Acad Sci USA 117(30):17513–17515
Molero-Salinas A, Rico-Luna C, Losada C, Buenestado-Serrano S, de la Cueva García VM, Egido J, Adán-Jiménez J, Catalán P, Muñoz P, Pérez-Lago L et al (2022) High SARS-CoV-2 viral load in travellers arriving in Spain with a negative COVID-19 test prior to departure. J Travel Med 29(3):180
Nicola M, Alsafi Z, Sohrabi C, Kerwan A, Al-Jabir A, Iosifidis C, Agha M, Agha R (2020) The socio-economic implications of the coronavirus pandemic (COVID-19): a review. Int J Surg 78:185–193
Robertson SL, Henson SM, Robertson T, Cushing J (2018) A matter of maturity: to delay or not to delay? Continuous-time compartmental models of structured populations in the literature 2000–2016. Nat Resour Model 31(1):12160
Russell TW, Wu JT, Clifford S, Edmunds WJ, Kucharski AJ, Jit M et al (2021) Effect of internationally imported cases on internal spread of COVID-19: a mathematical modelling study. Lancet Public Health 6(1):12–20
Vilches TN, Sah P, Abdollahi E, Moghadas SM, Galvani AP (2021) Importance of non-pharmaceutical interventions in the COVID-19 vaccination era: a case study of the seychelles. J Glob Health 11:03104
Vilches TN, Rafferty E, Wells CR, Galvani AP, Moghadas SM (2022) Economic evaluation of COVID-19 rapid antigen screening programs in the workplace. BMC Med. https://doi.org/10.1186/s12916-022-02641-5
Wells CR, Pandey A, Fitzpatrick MC, Crystal WS, Singer BH, Moghadas SM, Galvani AP, Townsend JP (2022) Quarantine and testing strategies to ameliorate transmission due to travel during the COVID-19 pandemic: a modelling study. Lancet Reg Health-Europe 100304
Wells CR, Sah P, Moghadas SM, Pandey A, Shoukat A, Wang Y, Wang Z, Meyers LA, Singer BH, Galvani AP (2020) Impact of international travel and border control measures on the global spread of the novel 2019 coronavirus outbreak. Proc Natl Acad Sci 117(13):7504–7509
Wells CR, Townsend JP, Pandey A, Moghadas SM, Krieger G, Singer B, McDonald RH, Fitzpatrick MC, Galvani AP (2021) Optimal COVID-19 quarantine and testing strategies. Nat Commun 12(1):1–9
Wells CR, Pandey A, Moghadas SM, Singer BH, Krieger G, Heron RJL, Turner DE, Abshire JP, Phillips KM, Donoghue AM, Galvani AP, Townsend JP (2022) Comparative analyses of eighteen rapid antigen tests and RT-PCR for COVID-19 quarantine and surveillance-based isolation. Commun Med. https://doi.org/10.1038/s43856-022-00147-y
Wells CR, Pandey A, Gokcebel S, Krieger G, Donoghue AM, Singer BH, Moghadas SM, Galvani AP, Townsend JP (2022) Quarantine and serial testing for variants of SARS-Cov-2 with benefits of vaccination and boosting on consequent control of COVID-19. PNAS Nexus. https://doi.org/10.1093/pnasnexus/pgac100
Xu Z, Zhao X-Q (2012) A vector-bias malaria model with incubation period and diffusion. Discrete Contin Dyn Syst Ser B 17(7):2615–2634
Funding
The research was partially supported by the Natural Sciences and Engineering Research Council of Canada, Discovery Grant; Canadian Institutes of Health Research (OV4-170643, COVID-19 Rapid Research), and NSERC-MfPH Grant for Emerging Infectious Disease Modelling.
Author information
Authors and Affiliations
Contributions
JX, SMM: conceptualized the study; JX, ZW: designed the model and performed the analysis; JX, ZW, SMM: wrote the paper. All authors approve the content and final draft submitted for publication.
Corresponding author
Ethics declarations
Conflict of interest
We declare that we have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
1.1 Reproduction numbers
To set a baseline for our analysis, we consider the two populations without any control measures, such as testing, isolation, or quarantine, and assume the same travel rate between \(P_A\) and \(P_B\). This simplifies our model into two populations with a susceptible-exposed-infectious-recovered structure, connected by dispersal. At the disease-free equilibrium (without any infection), all variables except \(S_A\) and \(S_B\) are zeros with \(\bar{S}_{A}=\bar{S}_{B}=N:= (N_A+N_B)/2\) when \(\delta _{_A}=\delta _{_B}\). The basic reproduction number is given by \( \mathcal {R}_0=\beta N/\gamma \).
When control measures are applied, \(\mathcal {R}_0\) may be reduced, which is characterized by the control reproduction number \(\mathcal {R}_c\). To calculate \(\mathcal {R}_c\) for model (6), we use the Next Generation Matrix (NGM) approach introduced in Xu and Zhao (2012). Let \(x_1, \ldots , x_{11}\) be the number of individuals in the \(E_A, E_{iA}, I_A, I_{iA}\), and \(E_B, E_{iB}, I_B, I_{iB}, I_{q1B}, E_{qB}, I_{q2B}\) classes, respectively. It then follows from the model (6) that the distribution of the remaining individuals at time \(t>0\) is
Thus, the total numbers of newly infected individuals in each class are
Similarly, we have
From the relationship between \((\bar{x}_1, \ldots , \bar{x}_{11})\) and \(x_1, \ldots , x_{11}\), we have the next generation operator \(\mathcal {M}\) (Matrix (9)), where
Since the reproduction number is defined as the spectral radius of \(\mathcal {M}\) (Xu and Zhao 2012), we obtain \(\mathcal {R}_c = (\rho (\mathcal {M}))^2\).
We note that the interventions of testing before departure from \(P_A\), quarantine and its duration upon arrival in \(P_B\), and testing at exit quarantine, have no significant effects on the control reproduction number (Fig. 5). However, the proportion of infectious individuals isolated within the population (\(\eta \)) reduces the reproduction number, leading to further delay in case importation and the outbreak within \(P_B\) with a lower peak and cumulative incidence.
1.2 Effect of dispersal rate on the reproduction number
See Fig. 5.
Control reproduction number as a function of dispersal rate. Solid and dashed lines (overlapped) correspond to scenarios without and with testing before departure from \(P_A\). The duration of quarantine is A, D 1 day; B, E 2 days; and C, F 3 days, without testing at exit from quarantine (A, B, C), and with testing at exit from quarantine (D, E, F) in \(P_B\)
1.3 Effect of dispersal rate on peak-time of the outbreak
See Fig. 6.
Range for the peak time of incidence in \(P_B\) when dispersal rate, \(\delta \), varies between 0.0001 and 0.001, with quarantine of travelers upon arrival. Solid and dashed lines correspond to scenarios without and with testing before departure from \(P_A\). The duration of quarantine is A, D 1 day; B, E 2 days; and C, F 3 days, without testing at exit from quarantine (A, B, C), and with testing at exit from quarantine (D, E, F)
1.4 Cumulative incidence
See Fig. 7.
Attack rate (i.e., the proportion of the population infected throughout the outbreak) in \(P_B\) when quarantine of travelers is implemented upon arrival. Solid and dashed lines correspond to scenarios without and with testing before departure from \(P_A\). The duration of quarantine is A, D 1 day; B, E 2 days; and C, F 3 days, without testing at exit from quarantine (A, B, C), and with testing at exit from quarantine (D, E, F)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, J., Wang, Z. & Moghadas, S.M. Modelling the effect of travel-related policies on disease control in a meta-population structure. J. Math. Biol. 87, 55 (2023). https://doi.org/10.1007/s00285-023-01990-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00285-023-01990-w