Abstract
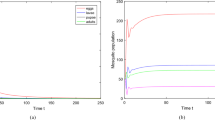
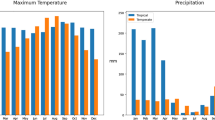
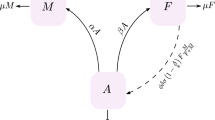
Diapause, a period of arrested development caused by adverse environmental conditions, serves as a key survival mechanism for insects and other invertebrate organisms in temperate and subtropical areas. In this paper, a novel modelling framework, motivated by mosquito species, is proposed to investigate the effects of diapause on seasonal population growth, where the diapause period is taken as an independent growth process, during which the population dynamics are completely different from that in the normal developmental and post-diapause periods. More specifically, the annual growth period is divided into three intervals, and the population dynamics during each interval are described by different sets of equations. We formulate two models of delay differential equations (DDE) to explicitly describe mosquito population growth with a single diapausing stage, either immature or adult. These two models can be further unified into one DDE model, on which the well-posedness of the solutions and the global stability of the trivial and positive periodic solutions in terms of an index \({\mathcal {R}}\) are analysed. The seasonal population abundances of two temperate mosquito species with different diapausing stages are simulated to identify the essential role on population persistence that diapause plays and predict that killing mosquitoes during the diapause period can lower but fail to prevent the occurrence of peak abundance in the following season. Instead, culling mosquitoes during the normal growth period is much more efficient to decrease the outbreak size. Our modelling framework may shed light on the diapause-induced variations in spatiotemporal distributions of different mosquito species.








Similar content being viewed by others
References
Alekseev VR, De Stasio B, Gilbert JJ (2007) Diapause in aquatic invertebrates: theory and human use. Springer, New York
Beck-Johnson LM, Nelson WA, Paaijmans KP, Read AF, Thomas MB, Bjrnstad ON (2013) The effect of temperature on Anopheles mosquito population dynamics and the potential for malaria transmission. PLoS One 8(11):e79276
Belozerov VN (1982) Diapause and biological rhythms in ticks. In: Obenchain FD, Galun R (eds) Physiology of ticks: current themes in tropical science. Pergamon Press, Oxford, pp 469–500
Beverton RJ, Holt SJ (2012) On the dynamics of exploited fish populations. Springer, New York
Bradshaw ME, Holzapfel CM (1990) Resource limitation, habitat segregation, and species interactions of British tree-hole mosquitoes in nature. Oecologia 90(2):227–237
Bradshaw WE (1976) Geography of photoperiodic response in diapausing mosquito. Nature 262:384–386
Brauer F, Castillo-Chavez C (2001) Mathematical models in population biology and epidemiology. Springer, New York
Buth JL, Brust RA, Ellis RA (1990) Development time, oviposition activity and onset of diapause in Culex tarsalis, Culex restuans and Culiseta inornata in southern Manitoba. J Am Mosq Control Assoc 6(1):55–63
Cailly P, Tran A, Balenghien T, L’Ambert G, Toty C, Ezanno P (2012) A climate-driven abundance model to assess mosquito control strategies. Ecol Model 227:7–17
Cruz-Pacheco G, Esteva L, Montaõ-Hirose JA, Vargas C (2005) Modelling the dynamics of West Nile virus. Bull Math Biol 67(6):1157–1172
Cushing JM (1998) An introduction to structured population dynamics. SIAM, Philadelphia
Daszak P, Cunningham AA, Hyatt AD (2000) Emerging infectious diseases of wildlife-threats to biodiversity and human health. Science 287(5452):443–449
Denlinger DL (2002) Regulation of diapause. Annu Rev Entomol 47(1):93–122
Denlinger DL, Armbruster PA (2014) Mosquito diapause. Annu Rev Entomol 59:73–93
Denlinger DL, Lee RE Jr (2010) Low temperature biology of insects. Cambridge University Press, Cambridge
Denlinger DL, Yocum GD, Rinehart JP, Gilbert LI (2004) Hormonal control of diapause. In: Gilbert LI (ed) Insect endocrinology. Academic, San Diego, CA, pp 430–463
Ewing DA, Cobbold CA, Purse BV, Nunn MA, White SM (2016) Modelling the effect of temperature on the seasonal population dynamics of temperate mosquitoes. J Theor Biol 400:65–79
Fang J, Gourley SA, Lou Y (2016) Stage-structured models of intra- and inter-specific competition within age classes. J Differ Equ 260(2):1918–1953
Flannagan RD, Tammariello SP, Joplin KH, Cikra-Ireland RA, Yocum GD, Denlinger DL (1998) Diapause-specific gene expression in pupae of the flesh fly Sarcophaga crassipalpis. Proc Natl Acad Sci USA 95(10):5616–5620
Gong H, DeGaetano AT, Harrington LC (2011) Climate-based models for West Nile Culex mosquito vectors in the Northeastern US. Int J Biometeorol 55(3):435–446
Gourley SA, Wu J (2006) Delayed non-local diffusive systems in biological invasion and disease spread. In: Nonlinear dynamics and evolution equations, American Mathematical Society, pp 137–200
Gurney W, Nisbet RM, Lawton JH (1983) The systematic formulation of tractable single-species models incorporating age structure. J Anim Ecol 52(2):479–495
Hahn DA, Denlinger DL (2011) Energetics of insect diapause. Annu Rev Entomol 56:103–121
Hanson SM (1995) Field overwinter survivorship of Aedes albopictus eggs in Japan. J Am Mosq Control Assoc 11(3):354–357
Hanson SM, Craig GB Jr (1994) Cold acclimation, diapause, and geographic origin affect cold hardiness in eggs of Aedes albopictus (Diptera: Culicidae). J Med Entomol 31(2):192–201
Hasegawa K (1957) The diapause hormone of the silkworm. Bombyx mori. Nature 179(4573):1300–1301
Hodek I, van Emden HF, Honek A (2012) Ecology and behaviour of the ladybird beetles (Coccinellidae). Wiley, Chichester
Jia P, Lu L, Chen X, Chen J, Guo L, Yu X, Liu Q (2016) A climate-driven mechanistic population model of Aedes albopictus with diapause. Parasit Vectors 9(1):175
Koštál V (2006) Eco-physiological phases of insect diapause. J Insect Physiol 52(2):113–127
Lakovic M, Poethke HJ, Hovestadt T (2015) Dispersal timing: emigration of insects living in patchy environments. PLoS One 10(7):e0128672
Laing JE, Corrigan JE (1995) Diapause induction and post-diapause emergence in Trichogramma minutum Riley (Hymenoptera: Trichogrammatidae): the role of host species, temperature, and photoperiod. Can Entomol 127(1):103–110
Leisnham PT, Sala LM, Juliano SA (2008) Geographic variation in adult survival and reproductive tactics of the mosquito Aedes albopictus. J Med Entomol 45(2):210–221
Liang X, Zhang L, Zhao X-Q, Basic reproduction ratios for periodic abstract functional differential equations (with application to a spatial model for Lyme disease), J Dyn Diff Equat.https://doi.org/10.1007/s10884-017-9601-7
Liu K, Lou Y, Wu J (2017) Analysis of an age structured model for tick populations subject to seasonal effects. J Diff Equ 263:2078–2112
Lou Y, Zhao X-Q (2010) A climate-based malaria transmission model with structured vector population. SIAM J Appl Math 70(6):2023–2044
Lou Y, Zhao X-Q (2017) A theoretical approach to understanding population dynamics with seasonal developmental durations. J Nonlinear Sci 27(2):573–603
Martin RH (1976) Nonlinear operators and differential equations in Banach spaces. Wiley, New York
Metz JA, Diekmann O (1986) The dynamics of physiologically structured populations. Springer, Berlin, Germany
Marini G, Guzzetta G, Baldacchino F, Arnoldi D, Montarsi F, Capelli G, Rizzoli A, Merler S, Rosà R (2017) The effect of interspecific competition on the temporal dynamics of Aedes albopictus and Culex pipiens. Parasit Vectors 10(1):102
Pawelek KA, Niehaus P, Salmeron C, Hager EJ, Hunt GJ (2014) Modelling dynamics of Culex pipiens complex populations and assessing abatement strategies for West Nile Virus. PLoS One 9(9):e108452
Pritchard G (1989) The roles of temperature and diapause in the life history of a temperate-zone dragonfly: Argia vivida (Odonata: Coenagrionidae). Ecol Entomol 14(1):99–108
Ragland GJ, Denlinger DL, Hahn DA (2010) Mechanisms of suspended animation are revealed by transcript profiling of diapause in the flesh fly. Proc Natl Acad Sci USA 107:14909–14914
Rinehart JP, Robich RM, Denlinger DL (2006) Enhanced cold and desiccation tolerance in diapausing adults of Culex pipiens, and a role for Hsp70 in response to cold shock but not as a component of the diapause program. J Med Entomol 43(4):713–722
Silver JB (2007) Mosquito ecology: field sampling methods. Springer, New York
Sim C, Denlinger DL (2008) Insulin signalling and FOXO regulate the overwintering diapause of the mosquito Culex pipiens. Proc Natl Acad Sci USA 105(18):6777–6781
Spielman A (2001) Structure and seasonality of nearctic Culex pipiens populations. Ann NY Acad Sci 951(1):220–234
Smith DL, Perkins TA, Reiner RC Jr, Barker CM, Niu T, Chaves LF, Ellis AM, George DB, Le Menach A, Pulliam J et al (2014) Recasting the theory of mosquito-borne pathogen transmission dynamics and control. Trans R Soc Trop Med Hyg 108(4):185–197
Smith HL (1993) Reduction of structured population models to threshold-type delay equations and functional differential equations: a case study. Math Biosci 113(1):1–23
Smith HL (2010) An introduction to delay differential equations with applications to the life sciences. Springer, New York
Tauber MJ, Tauber CA (1976) Insect seasonality: diapause maintenance, termination, and postdiapause development. Annu Rev Entomol 21(1):81–107
Tauber MJ, Tauber CA, Masaki S (1986) Seasonal adaptations of insects. Oxford University Press, Oxford
Toma L, Severini F, Di Luca M, Bella A, Romi R (2003) Seasonal patterns of oviposition and egg hatching rate of Aedes albopictus in Rome. J Am Mosq Control Assoc 19(1):19–22
Tran A, L’Ambert G, Lacour G, Benoît R, Demarchi M, Cros M, Cailly P, Aubry-Kientz M, Balenghien T, Ezanno P (2013) A rainfall-and temperature-driven abundance model for Aedes albopictus populations. Int J Environ Res Public Health 10(5):1698–1719
Tsurim I, Silberbush A, Ovadia O, Blaustein L, Margalith Y (2013) Inter-and intra-specific density-dependent effects on life history and development strategies of larval mosquitoes. PLoS ONE 8(3):e57875
Vinogradova EB (2007) Diapause in aquatic insects, with emphasis on mosquitoes. In: Alekseev VR, De Stasio B, Gilbert JJ (eds) Diapause in aquatic invertebrates. Springer, London, pp 83–113
Wang X, Zhao X-Q (2017) A malaria transmission model with temperature-dependent incubation period. Bull Math Biol 79(5):1155–1182
Wang X, Zou X (2018) Threshold dynamics of a temperature-dependent stage-structured mosquito population model with nested delays. Bull Math Biol 80(7):1962–1987
Wu X, Magpantay FMG, Wu J, Zou X (2015) Stage-structured population systems with temporally periodic delay. Math Methods Appl Sci 38:3464–3481
Zhang X, Wu X, Wu J (2017) Critical contact rate for vector-host-pathogen oscillation involving co-feeding and diapause. J Biol Syst 25:657–675
Zhao X-Q (2017) Basic reproduction ratios for periodic compartmental models with time delay. J Dyn Diff Equat 29:67–82
Zhao X-Q (2017) Dynamical systems in population biology. Springer, New York
Zhao X-Q, Jing ZJ (1996) Global asymptotic behaviour in some cooperative systems of functional differential equations. Can Appl Math Q 4(4):421–444
Acknowledgements
The work of Y. L. and K. L. was supported in part by the Research Grants Council (PolyU 153277/16P) of Hong Kong. D. G. was supported by NSFC (11601336), Program for Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning (TP2015050), and Shanghai Gaofeng Project for University Academic Development Program. S. R. was supported by National Science Foundation (DMS-1853622) and Centre for Disease Control and Prevention “Southeastern Regional Centre of Excellence in Vector-Borne Diseases: The Gateway Program” (U01CK000510). The authors would like to thank Dr. Hongying Shu for helpful discussions on this paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lou, Y., Liu, K., He, D. et al. Modelling diapause in mosquito population growth. J. Math. Biol. 78, 2259–2288 (2019). https://doi.org/10.1007/s00285-019-01343-6
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00285-019-01343-6