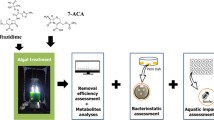
Abstract
Antibiotic pollution poses a potential risk of genotoxicity, as antibiotics released into the environment can induce DNA damage and mutagenesis in various organisms. This pollution, stemming from pharmaceutical manufacturing, agriculture, and improper disposal, can disrupt aquatic ecosystems and potentially impact human health through the consumption of contaminated water and food. The removal of genotoxic antibiotics using algae-mediated approaches has gained considerable attention due to its potential for mitigating the environmental and health risks associated with these compounds. The paper provides an in-depth examination of the molecular aspects concerning algae and bioreactor-driven methodologies utilized for the elimination of deleterious antibiotics. The molecular analysis encompasses diverse facets, encompassing the discernment and profiling of algae species proficient in antibiotic degradation, the explication of enzymatic degradation pathways, and the refinement of bioreactor configurations to augment removal efficacy. Emphasizing the significance of investigating algal approaches for mitigating antibiotic pollution, this paper underscores their potential as a sustainable solution, safeguarding both the environment and human health.


Similar content being viewed by others
References
Mulchandani R, Wang Y, Gilbert M, Van Boeckel TP (2023) Global trends in antimicrobial use in food-producing animals: 2020 to 2030. PLOS Global Public Health 3:e0001305
Mutuku C, Gazdag Z, Melegh S (2022) Occurrence of antibiotics and bacterial resistance genes in wastewater: resistance mechanisms and antimicrobial resistance control approaches. World J Microbiol Biotechnol 38:152. https://doi.org/10.1007/s11274-022-03334-0
Karkman A, Pärnänen K, Larsson DGJ (2019) Fecal pollution can explain antibiotic resistance gene abundances in anthropogenically impacted environments. Nat Commun 10:80. https://doi.org/10.1038/s41467-018-07992-3
Patangia DV, Anthony Ryan C, Dempsey E et al (2022) Impact of antibiotics on the human microbiome and consequences for host health. Microbiologyopen 11:e1260. https://doi.org/10.1002/mbo3.1260
Kitamura RSA, Vicentini M, Bitencourt V et al (2023) Salvinia molesta phytoremediation capacity as a nature-based solution to prevent harmful effects and accumulation of ciprofloxacin in Neotropical catfish. Environ Sci Pollut Res Int 30:41848–41863. https://doi.org/10.1007/s11356-023-25226-y
Mottola F, Iovine C, Santonastaso M et al (2022) Evaluation of zebrafish DNA integrity after individual and combined exposure to TiO2 nanoparticles and lincomycin. Toxics 10:132. https://doi.org/10.3390/toxics10030132
Bojarski B, Kot B, Witeska M (2020) Antibacterials in aquatic environment and their toxicity to fish. Pharmaceuticals (Basel) 13:189. https://doi.org/10.3390/ph13080189
Chen N, Zhang X, Du Q et al (2023) Advancements in swine wastewater treatment: removal mechanisms, influential factors, and optimization strategies. J Water Process Eng 54:103986
Ciğeroğlu Z, Kazan-Kaya ES, El Messaoudi N et al (2023) Remediation of tetracycline from aqueous solution through adsorption on g-C3N4-ZnO-BaTiO3 nanocomposite: optimization, modeling, and theoretical calculation. J Mol Liq 369:120866. https://doi.org/10.1016/j.molliq.2022.120866
Prabhu SM, Yusuf M, Ahn Y et al (2023) Fluoride occurrence in environment, regulations, and remediation methods for soil: a comprehensive review. Chemosphere. https://doi.org/10.1016/j.chemosphere.2023.138334
Obaid ZH, Salman JM, Kadhim NF (2023) Review on toxicity and removal of pharmaceutical pollutants using immobilised microalgae. Ecol Eng Environ Technol. https://doi.org/10.12912/27197050/166013
Akhtar N, Wani AK, Singh R et al (2023) Chapter 33—Bioprospecting microalgae for biofuel synthesis: a gateway to sustainable energy. In: Shah MP (ed) Green approach to alternative fuel for a sustainable future. Elsevier, Amsterdam, pp 453–462
Hanna N, Tamhankar AJ, Stålsby Lundborg C (2023) Antibiotic concentrations and antibiotic resistance in aquatic environments of the WHO Western Pacific and South-East Asia regions: a systematic review and probabilistic environmental hazard assessment. Lancet Planet Health 7:e45–e54. https://doi.org/10.1016/S2542-5196(22)00254-6
Van Boeckel TP, Brower C, Gilbert M et al (2015) Global trends in antimicrobial use in food animals. Proc Natl Acad Sci USA 112:5649–5654. https://doi.org/10.1073/pnas.1503141112
Browne AJ, Chipeta MG, Haines-Woodhouse G et al (2021) Global antibiotic consumption and usage in humans, 2000–18: a spatial modelling study. Lancet Planet Health 5:e893–e904. https://doi.org/10.1016/S2542-5196(21)00280-1
Arun S, Xin L, Gaonkar O et al (2022) Antibiotics in sewage treatment plants, receiving water bodies and groundwater of Chennai city and the suburb, South India: occurrence, removal efficiencies, and risk assessment. Sci Total Environ 851:158195. https://doi.org/10.1016/j.scitotenv.2022.158195
Gao L, Shi Y, Li W et al (2012) Occurrence of antibiotics in eight sewage treatment plants in Beijing, China. Chemosphere 86:665–671. https://doi.org/10.1016/j.chemosphere.2011.11.019
Li L, He J, Gan Z, Yang P (2021) Occurrence and fate of antibiotics and heavy metals in sewage treatment plants and risk assessment of reclaimed water in Chengdu, China. Chemosphere 272:129730. https://doi.org/10.1016/j.chemosphere.2021.129730
Oliveira TS, Murphy M, Mendola N et al (2015) Characterization of pharmaceuticals and personal care products in hospital effluent and waste water influent/effluent by direct-injection LC-MS-MS. Sci Total Environ 518–519:459–478. https://doi.org/10.1016/j.scitotenv.2015.02.104
Han QF, Zhao S, Zhang XR et al (2020) Distribution, combined pollution and risk assessment of antibiotics in typical marine aquaculture farms surrounding the Yellow Sea, North China. Environ Int 138:105551. https://doi.org/10.1016/j.envint.2020.105551
Han QF, Song C, Sun X et al (2021) Spatiotemporal distribution, source apportionment and combined pollution of antibiotics in natural waters adjacent to mariculture areas in the Laizhou Bay, Bohai Sea. Chemosphere 279:130381. https://doi.org/10.1016/j.chemosphere.2021.130381
Wang C, Mao Y, Zhou W et al (2023) Inhomogeneous antibiotic distribution in sediment profiles in anthropogenically impacted lakes: source apportionment, fate drivers, and risk assessment. J Environ Manage 341:118048. https://doi.org/10.1016/j.jenvman.2023.118048
Liu C, Tan L, Zhang L et al (2021) A review of the distribution of antibiotics in water in different regions of China and current antibiotic degradation pathways. Front Environ Sci 9:692298
Liu Y, Feng M, Wang B et al (2020) Distribution and potential risk assessment of antibiotic pollution in the main drinking water sources of Nanjing, China. Environ Sci Pollut Res Int 27:21429–21441. https://doi.org/10.1007/s11356-020-08516-7
Deng WJ, Li N, Ying GG (2018) Antibiotic distribution, risk assessment, and microbial diversity in river water and sediment in Hong Kong. Environ Geochem Health 40:2191–2203. https://doi.org/10.1007/s10653-018-0092-1
Wang K, Zhuang T, Su Z et al (2021) Antibiotic residues in wastewaters from sewage treatment plants and pharmaceutical industries: occurrence, removal and environmental impacts. Sci Total Environ 788:147811. https://doi.org/10.1016/j.scitotenv.2021.147811
Grenni P, Ancona V, Barra Caracciolo A (2018) Ecological effects of antibiotics on natural ecosystems: a review. Microchem J 136:25–39. https://doi.org/10.1016/j.microc.2017.02.006
Manyi-Loh C, Mamphweli S, Meyer E, Okoh A (2018) Antibiotic use in agriculture and its consequential resistance in environmental sources: potential public health implications. Molecules 23:795. https://doi.org/10.3390/molecules23040795
Hu F, Dong F, Yin L et al (2021) Effects of sulfamethoxazole on the growth, oxidative stress and inflammatory response in the liver of juvenile Nile tilapia (Oreochromis niloticus). Aquaculture 543:736935. https://doi.org/10.1016/j.aquaculture.2021.736935
Islam MT, Mostakim GM, Azom MG et al (2022) Effect of an amalgamated antibiotic and its connection to cyto-genotoxicity and histo-architectural malformations in stinging catfish. Emerg Contam 8:381–390. https://doi.org/10.1016/j.emcon.2022.09.001
Jia D, You X, Tang M et al (2023) Single and combined genotoxicity of metals and fluoroquinolones to zebrafish embryos at environmentally relevant concentrations. Aquat Toxicol 258:106495. https://doi.org/10.1016/j.aquatox.2023.106495
Rodrigues S, Antunes SC, Correia AT, Nunes B (2017) Rainbow trout (Oncorhynchus mykiss) pro-oxidant and genotoxic responses following acute and chronic exposure to the antibiotic oxytetracycline. Ecotoxicology 26:104–117. https://doi.org/10.1007/s10646-016-1746-3
Zhao XL, Li P, Zhang SQ et al (2021) Effects of environmental norfloxacin concentrations on the intestinal health and function of juvenile common carp and potential risk to humans. Environ Pollut 287:117612. https://doi.org/10.1016/j.envpol.2021.117612
Rodrigues S, Antunes SC, Correia AT et al (2019) Assessment of toxic effects of the antibiotic erythromycin on the marine fish gilthead seabream (Sparus aurata L.) by a multi-biomarker approach. Chemosphere 216:234–247. https://doi.org/10.1016/j.chemosphere.2018.10.124
Fu J, Gong Z, Kelly BC (2019) Metabolomic profiling of zebrafish (Danio rerio) embryos exposed to the antibacterial agent triclosan. Environ Toxicol Chem 38:240–249. https://doi.org/10.1002/etc.4292
Jerbi MA, Ouanes Z, Besbes R et al (2011) Single and combined genotoxic and cytotoxic effects of two xenobiotics widely used in intensive aquaculture. Mutat Res 724:22–27. https://doi.org/10.1016/j.mrgentox.2011.04.010
Mhadhbi L, El Ayari T, Tir M, Kadri D (2022) Azithromycin effects on the European sea bass (Dicentrarchus labrax) early life stages following acute and chronic exposure: laboratory bioassays. Drug Chem Toxicol 45:1295–1301. https://doi.org/10.1080/01480545.2020.1822388
Bernier SP, Surette MG (2013) Concentration-dependent activity of antibiotics in natural environments. Front Microbiol 4:20. https://doi.org/10.3389/fmicb.2013.00020
Alegun O, Pandeya A, Cui J et al (2021) Donnan potential across the outer membrane of gram-negative bacteria and its effect on the permeability of antibiotics. Antibiotics (Basel) 10:701. https://doi.org/10.3390/antibiotics10060701
Gojkovic Z, Lindberg RH, Tysklind M, Funk C (2019) Northern green algae have the capacity to remove active pharmaceutical ingredients. Ecotoxicol Environ Saf 170:644–656. https://doi.org/10.1016/j.ecoenv.2018.12.032
Hena S, Gutierrez L, Croué JP (2020) Removal of metronidazole from aqueous media by C. vulgaris. J Hazard Mater 384:121400. https://doi.org/10.1016/j.jhazmat.2019.121400
Daneshvar E, Zarrinmehr MJ, Hashtjin AM et al (2018) Versatile applications of freshwater and marine water microalgae in dairy wastewater treatment, lipid extraction and tetracycline biosorption. Bioresour Technol 268:523–530. https://doi.org/10.1016/j.biortech.2018.08.032
Qian Z, Na L, Bao-Long W et al (2022) Capabilities and mechanisms of microalgae on nutrients and florfenicol removing from marine aquaculture wastewater. J Environ Manage 320:115673. https://doi.org/10.1016/j.jenvman.2022.115673
Han K, Liu Y, Hu J et al (2022) Effect of live and inactivated Chlamydomonas reinhardtii on the removal of tetracycline in aquatic environments. Chemosphere 309:136666. https://doi.org/10.1016/j.chemosphere.2022.136666
Guo WQ, Zheng HS, Li S et al (2016) Removal of cephalosporin antibiotics 7-ACA from wastewater during the cultivation of lipid-accumulating microalgae. Biores Technol 221:284–290. https://doi.org/10.1016/j.biortech.2016.09.036
Liu K, Li J, Zhou Y et al (2023) Combined toxicity of erythromycin and roxithromycin and their removal by Chlorella pyrenoidosa. Ecotoxicol Environ Saf 257:114929. https://doi.org/10.1016/j.ecoenv.2023.114929
Yu Y, Zhou Y, Wang Z et al (2017) Investigation of the removal mechanism of antibiotic ceftazidime by green algae and subsequent microbic impact assessment. Sci Rep 7:4168. https://doi.org/10.1038/s41598-017-04128-3
Ricky R, Chiampo F, Shanthakumar S (2022) Efficacy of ciprofloxacin and amoxicillin removal and the effect on the biochemical composition of Chlorella vulgaris. Bioengineering (Basel) 9:134. https://doi.org/10.3390/bioengineering9040134
Nguyen TB, Truong QM, Chen CW et al (2022) Pyrolysis of marine algae for biochar production for adsorption of Ciprofloxacin from aqueous solutions. Bioresour Technol 351:127043. https://doi.org/10.1016/j.biortech.2022.127043
Xiong JQ, Cui P, Ru S (2020) Biodegradation of Doxylamine from wastewater by a green microalga, Scenedesmus obliquus. Front Microbiol 11:584020. https://doi.org/10.3389/fmicb.2020.584020
Song C, Wei Y, Qiu Y et al (2019) Biodegradability and mechanism of florfenicol via Chlorella sp. UTEX1602 and L38: experimental study. Bioresour Technol 272:529–534. https://doi.org/10.1016/j.biortech.2018.10.080
Jiang R, Wei Y, Sun J et al (2019) Degradation of cefradine in alga-containing water environment: a mechanism and kinetic study. Environ Sci Pollut Res Int 26:9184–9192. https://doi.org/10.1007/s11356-019-04279-y
Mojiri A, Baharlooeian M, Zahed MA (2021) The potential of Chaetoceros muelleri in bioremediation of antibiotics: performance and optimization. Int J Environ Res Public Health 18:977. https://doi.org/10.3390/ijerph18030977
Baquero F, Coque TM, Martínez JL (2022) Natural detoxification of antibiotics in the environment: a one health perspective. Front Microbiol 13:1062399
Peng J, Cao KL, Lv SB et al (2023) Algal strains, treatment systems and removal mechanisms for treating antibiotic wastewater by microalgae. J Water Process Eng 56:104266
Roy B, Suresh PK, Chandrasekaran N, Mukherjee A (2021) Antibiotic tetracycline enhanced the toxic potential of photo catalytically active P25 titanium dioxide nanoparticles towards freshwater algae Scenedesmus obliquus. Chemosphere 267:128923. https://doi.org/10.1016/j.chemosphere.2020.128923
Xiong J-Q, Kurade MB, Patil DV et al (2017) Biodegradation and metabolic fate of levofloxacin via a freshwater green alga, Scenedesmus obliquus in synthetic saline wastewater. Algal Res 25:54–61. https://doi.org/10.1016/j.algal.2017.04.012
Qi X, Ru S, Xiong JQ (2022) Ecotoxicological effects of sulfacetamide on a green microalga, Desmodesmus quadricauda: cell viability, antioxidant system, and biotransformation. Environ Technol Innov 26:102278. https://doi.org/10.1016/j.eti.2022.102278
Hom-Diaz A, Jaén-Gil A, Rodríguez-Mozaz S et al (2022) Insights into removal of antibiotics by selected microalgae (Chlamydomonas reinhardtii, Chlorella sorokiniana, Dunaliella tertiolecta and Pseudokirchneriella subcapitata). Algal Res 61:102560. https://doi.org/10.1016/j.algal.2021.102560
Giraldo AL, Erazo-Erazo ED, Flórez-Acosta OA et al (2015) Degradation of the antibiotic oxacillin in water by anodic oxidation with Ti/IrO2 anodes: evaluation of degradation routes, organic by-products and effects of water matrix components. Chem Eng J 279:103–114
Wani AK, Akhtar N, Sher F et al (2022) Microbial adaptation to different environmental conditions: molecular perspective of evolved genetic and cellular systems. Arch Microbiol 204:144
Qixin L, Xuan F, Zhiya S et al (2022) Enhanced wastewater treatment performance by understanding the interaction between algae and bacteria based on quorum sensing. Bioresour Technol 354:127161. https://doi.org/10.1016/j.biortech.2022.127161
Zhou Y, Li X, Chen J, Wang F (2023) Treatment of antibiotic-containing wastewater with self-suspended algae-bacteria symbiotic particles: removal performance and reciprocal mechanism. Chemosphere 323:138240. https://doi.org/10.1016/j.chemosphere.2023.138240
Zambrano J, García-Encina PA, Hernández F et al (2023) Kinetics of the removal mechanisms of veterinary antibiotics in synthetic wastewater using microalgae–bacteria consortia. Environ Technol Innov 29:103031. https://doi.org/10.1016/j.eti.2023.103031
Rahayu F, Wani AK, Murianingrum M et al (2022) Studies on dew retting process of kenaf by formulation of indigenous consortium bacteria. AIP Conf Proc 2454:060041. https://doi.org/10.1063/5.0078708
Roh H, Subramanya N, Zhao F et al (2009) Biodegradation potential of wastewater micropollutants by ammonia-oxidizing bacteria. Chemosphere 77:1084–1089. https://doi.org/10.1016/j.chemosphere.2009.08.049
Karigar CS, Rao SS (2011) Role of microbial enzymes in the bioremediation of pollutants: a review. Enzyme Res. https://doi.org/10.4061/2011/805187
Eheneden I, Wang R, Zhao J (2023) Antibiotic removal by microalgae-bacteria consortium: metabolic pathways and microbial responses. Sci Total Environ 891:164489. https://doi.org/10.1016/j.scitotenv.2023.164489
Ghattas A-K, Fischer F, Wick A, Ternes TA (2017) Anaerobic biodegradation of (emerging) organic contaminants in the aquatic environment. Water Res 116:268–295. https://doi.org/10.1016/j.watres.2017.02.001
Rong H, Li Y, Wang J et al (2023) Towards advanced mariculture wastewater treatment by bacterial-algal symbiosis system with different bacteria and algae inoculation ratios. J Water Process Eng 53:103826. https://doi.org/10.1016/j.jwpe.2023.103826
Wei J, Wang Z, Zhao C et al (2023) Effect of GR24 concentrations on tetracycline and nutrient removal from biogas slurry by different microalgae-based technologies. Bioresour Technol 369:128400. https://doi.org/10.1016/j.biortech.2022.128400
Tang Y, Song L, Ji X et al (2022) Algal-bacterial consortium mediated system offers effective removal of nitrogen nutrients and antibiotic resistance genes. Biores Technol 362:127874. https://doi.org/10.1016/j.biortech.2022.127874
Wang Y, Li J, Lei Y et al (2022) Enhanced sulfonamides removal via microalgae-bacteria consortium via co-substrate supplementation. Biores Technol 358:127431. https://doi.org/10.1016/j.biortech.2022.127431
Wang Y, Gong X, Huang D, Zhang J (2022) Increasing oxytetracycline and enrofloxacin concentrations on the algal growth and sewage purification performance of an algal-bacterial consortia system. Chemosphere 286:131917. https://doi.org/10.1016/j.chemosphere.2021.131917
Wang Y, Li J, Lei Y et al (2022) Bioremediation of sulfonamides by a microalgae-bacteria consortium—analysis of pollutants removal efficiency, cellular composition, and bacterial community. Bioresour Technol 351:126964. https://doi.org/10.1016/j.biortech.2022.126964
Wang Y, He Y, Li X et al (2022) Enhanced biodegradation of chlortetracycline via a microalgae-bacteria consortium. Biores Technol 343:126149. https://doi.org/10.1016/j.biortech.2021.126149
da Silva Rodrigues DA, da Cunha CCRF, do Santo DR et al (2021) Removal of cephalexin and erythromycin antibiotics, and their resistance genes, by microalgae-bacteria consortium from wastewater treatment plant secondary effluents. Environ Sci Pollut Res Int 28:67822–67832. https://doi.org/10.1007/s11356-021-15351-x
da Silva Rodrigues DA, da Cunha CCRF, Freitas MG et al (2020) Biodegradation of sulfamethoxazole by microalgae-bacteria consortium in wastewater treatment plant effluents. Sci Total Environ 749:141441. https://doi.org/10.1016/j.scitotenv.2020.141441
Qin L, Zhang Y, Xu Z, Zhang G (2018) Advanced membrane bioreactors systems: New materials and hybrid process design. Biores Technol 269:476–488
Mojiri A, Zhou JL, KarimiDermani B et al (2023) Anaerobic membrane bioreactor (AnMBR) for the removal of dyes from water and wastewater: progress, challenges, and future perspectives. Processes 11:855
Soroosh H, Otterpohl R, Hanelt D (2023) Influence of supplementary carbon on reducing the hydraulic retention time in microalgae-bacteria (MaB) treatment of municipal wastewater. J Water Process Eng 51:103447
Carneiro RB, Pozzi E, Corbi JJ, Zaiat M (2021) Ecotoxicity and antimicrobial inhibition assessment of effluent from an anaerobic bioreactor applied to the removal of sulfamethoxazole and ciprofloxacin antibiotics from domestic sewage. Water Air Soil Pollut 232:143. https://doi.org/10.1007/s11270-021-05097-0
Ruas G, López-Serna R, Scarcelli PG et al (2022) Influence of the hydraulic retention time on the removal of emerging contaminants in an anoxic-aerobic algal-bacterial photobioreactor coupled with anaerobic digestion. Sci Total Environ 827:154262. https://doi.org/10.1016/j.scitotenv.2022.154262
Oliveira AS, Alves M, Leitão F et al (2023) Bioremediation of coastal aquaculture effluents spiked with florfenicol using microalgae-based granular sludge—a promising solution for recirculating aquaculture systems. Water Res 233:119733. https://doi.org/10.1016/j.watres.2023.119733
Aydin S, Ünlü İD, Arabacı DN, Duru ÖA (2022) Evaluating the effect of microalga Haematococcus pluvialis bioaugmentation on aerobic membrane bioreactor in terms of performance, membrane fouling and microbial community structure. Sci Total Environ 807:149908. https://doi.org/10.1016/j.scitotenv.2021.149908
Escapa C, Torres T, Neuparth T et al (2018) Zebrafish embryo bioassays for a comprehensive evaluation of microalgae efficiency in the removal of diclofenac from water. Sci Total Environ 640–641:1024–1033. https://doi.org/10.1016/j.scitotenv.2018.05.353
Hom-Diaz A, Jaén-Gil A, Bello-Laserna I et al (2017) Performance of a microalgal photobioreactor treating toilet wastewater: pharmaceutically active compound removal and biomass harvesting. Sci Total Environ 592:1–11. https://doi.org/10.1016/j.scitotenv.2017.02.224
Gentili FG, Fick J (2017) Algal cultivation in urban wastewater: an efficient way to reduce pharmaceutical pollutants. J Appl Phycol 29:255–262. https://doi.org/10.1007/s10811-016-0950-0
Ismail MM, Essam TM, Ragab YM et al (2017) Remediation of a mixture of analgesics in a stirred-tank photobioreactor using microalgal-bacterial consortium coupled with attempt to valorise the harvested biomass. Biores Technol 232:364–371. https://doi.org/10.1016/j.biortech.2017.02.062
Ismail MM, Essam TM, Ragab YM, Mourad FE (2016) Biodegradation of ketoprofen using a microalgal-bacterial consortium. Biotechnol Lett 38:1493–1502. https://doi.org/10.1007/s10529-016-2145-9
Chen J, Zheng F, Guo R (2015) Algal feedback and removal efficiency in a sequencing batch reactor algae process (SBAR) to treat the antibiotic cefradine. PLoS ONE 10:e0133273. https://doi.org/10.1371/journal.pone.0133273
Mofijur M, Hasan M, Sultana S et al (2023) Advancements in algal membrane bioreactors: overcoming obstacles and harnessing potential for eliminating hazardous pollutants from wastewater. Chemosphere. https://doi.org/10.1016/j.chemosphere.2023.139291
Muth-Pawlak D, Kreula S, Gollan PJ et al (2022) Patterning of the autotrophic, mixotrophic, and heterotrophic proteomes of oxygen-evolving cyanobacterium Synechocystis sp. PCC 6803. Front Microbiol 13:891895. https://doi.org/10.3389/fmicb.2022.891895
Xiao Z, Zheng Y, Gudi CR et al (2021) Development of a kinetic model to describe six types of symbiotic interactions in a formate utilizing microalgae-bacteria cultivation system. Algal Res 58:102372
Kiki C, Ye X, Li X et al (2022) Continuous antibiotic attenuation in algal membrane photobioreactor: performance and kinetics. J Hazard Mater 434:128910. https://doi.org/10.1016/j.jhazmat.2022.128910
Peng YY, Gao F, Yang HL et al (2020) Simultaneous removal of nutrient and sulfonamides from marine aquaculture wastewater by concentrated and attached cultivation of Chlorella vulgaris in an algal biofilm membrane photobioreactor (BF-MPBR). Sci Total Environ 725:138524. https://doi.org/10.1016/j.scitotenv.2020.138524
Gao F, Zhou JL, Zhang YR et al (2023) Efficient coupling of sulfadiazine removal with microalgae lipid production in a membrane photobioreactor. Chemosphere 316:137880. https://doi.org/10.1016/j.chemosphere.2023.137880
López-Serna R, García D, Bolado S et al (2019) Photobioreactors based on microalgae-bacteria and purple phototrophic bacteria consortia: a promising technology to reduce the load of veterinary drugs from piggery wastewater. Sci Total Environ 692:259–266. https://doi.org/10.1016/j.scitotenv.2019.07.126
Wani AK, Chopra C, Singh R et al (2023) Mining microbial tapestry using high-throughput sequencing and In silico analysis of Trehalose synthase (TreS) derived from hot spring metagenome. Biocatal Agric Biotechnol 52:102829. https://doi.org/10.1016/j.bcab.2023.102829
Wani AK, Ahmad S, Américo-Pinheiro JHP et al (2023) Building the taxonomic profile of the Riniaie Marwah hot spring of Kishtwar in Jammu and Kashmir: the first high-throughput sequencing-based metagenome study. Iran J Microbiol 15:723–733
Author information
Authors and Affiliations
Contributions
AKW: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Resources; Software; Validation; Visualization; Roles/Writing—original draft; Writing—review and editing. TGM, NA, CC: Conceptualization; Validation; Visualization; Roles/Writing—original draft. SMB, SH: Visualization; Roles/Writing—original draft. VK: Visualization, Reviewing and Editing. RS: Investigation; Methodology; Resources; Software; Validation; Visualization; Roles/Writing—original draft; Supervision. JHPAP: Formal analysis, Software, Resources, Writing—original draft, Administration, Supervision, Submission, Reviewing and Editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical Approval
Not applicable.
Informed Consent
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wani, A.K., ul Gani Mir, T., Akhtar, N. et al. Algae-Mediated Removal of Prevalent Genotoxic Antibiotics: Molecular Perspective on Algae-Bacteria Consortia and Bioreactor-Based Strategies. Curr Microbiol 81, 112 (2024). https://doi.org/10.1007/s00284-024-03631-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-024-03631-x