Abstract
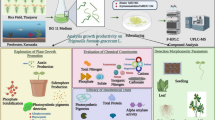
Synthesizing nanoparticles through a green synthesis approach is common nowadays. Cyanobacteria have attained great importance in the field of biosynthesis of nanoparticles as there is no use of toxic chemicals as reducing or capping agents for the synthesis of metal oxide nanoparticles. Micronutrient-based nano-formulations have become a topic of great interest in recent times due to their various advantageous properties and applications in agriculture. The current study aims to exploit the potential cyanobacterial strains isolated from different locations such as freshwater and soil ecosystems. The potential cyanobacterial isolates were screened based on their multiple plant growth promoting (PGP) attributes such as Indol acetic acid (IAA) production, siderophores, and phosphate solubilization. After the screening of cyanobacteria based on multiple PGP activities, the cyanobacterial strain was identified at the species level as Pseudanabaena foetida RJ1, based on microscopy and molecular characterization using 16S rRNA gene sequencing. The cyanobacterial biomass extract and cell-free extracts are utilized for the synthesis of CuO micronutrient Nanoparticles (NPs). The cyanobacterial strain Pseudanabaena foetida RJ1 possesses plant growth-promoting (PGP) attributes that provide reduction and capping for CuO NPs. The synthesized NPs were characterized and subjected to make a nano-formulation, utilizing the cyanobacteria-mediated CuO NPs along with low-cost zeolite as an adsorbent. The application of cyanobacterial biomass extract and cell-free extract provided an excellent comparative aspect in terms of micronutrient NP synthesis. The NPs in the form of formulations were applied to germinated paddy seeds (Pusa Basmati -1509) with varying concentrations (5, 10, 15 mg/l). Effects of cyanobacteria based CuO NPs on hydroponically grown paddy crops were analyzed. The application of nano-formulations has shown a significant increase in plant growth promotion in rice plants under hydroponics conditions. There is no such type of comparative investigation reported earlier, and NPs of micronutrients can be utilized as a new economic nanofertilizer and can be applied to plants for their growth promotion.





Similar content being viewed by others
References
De França Bettencourt GM, Degenhardt J, Zevallos Torres LA, de Andrade Tanobe VO, Soccol CR (2020) Green biosynthesis of single and bimetallic nanoparticles of iron and manganese using bacterial auxin complex to act as plant bio-fertilizer. Biocat and Agri Biotech 30:1–26
Calabi-Floody M, Medina J, Rumpel C, Condron LM, Hernandez M, Dumont M, de la Mora ML (2018) Smart fertilizers as a strategy for sustainable agriculture. Adv Agron 10(003):119–157
Tombuloglu H, Ercan I, Alshammari T, Tombuloglu G, Slimani Y, Almessiere M, Baykal A (2020) Incorporation of micro-nutrients (nickel, copper, zinc, and iron) into plant body through nanoparticles. J of Soil Sci and Plant Nutrition. https://doi.org/10.1007/s42729-020-00258-2
Wairich A, De Conti L, Lamb TI, Keil R, Neves LO, Brunetto G, Sperotto RA, Ricachenevsky FK (2022) Throwing copper around: how plants control uptake, distribution, and accumulation of copper. Agronomy 12:994
Khan I, Saeed K, Khan I (2019) Nanoparticles: properties, applications and toxicities. Arab J Chem 12:908–931
Ijaz I, Gilani E, Nazir A, Bukhari A (2020) Detail review on chemical, physical and green synthesis, classification, characterizations and applications of nanoparticles. Green Chem Lett Rev 13(3):223–245
Rahman A, Ismail A, Jumbianti D, Magdalena S, Sudrajat H (2009) Synthesis of copper oxide nano particles by using cyanobacterium. Indo J Chem 9(3):355–360
Bhardwaj B, Singh P, Kumar A, Kumar S, Budhwar V (2020) Eco-friendly greener synthesis of nanoparticles. Adv Pharm Bull 10(4):566–576
Khazi MI, Li C, Liaqat F, Malec P, Li J, Fu P (2021) Acclimation and characterization of marine cyanobacterial strains euryhalinema and desertifilum for C-phycocyanin production. Front Bioeng Biotechnol 9:752024
Hamida RS, Ali MA, Redhwan A, Bin-Meferij MM (2020) Cyanobacteria—a promising platform in green nanotechnology: a review on nanoparticles fabrication and their prospective applications. Intern J Nanomed 15:6033–6066
Kumar M, Saxena R, Parihar SS, Rai PK, Tomar RS (2018) Molecular characterization and phylogeny of some cyanobacterial strains isolated from soil and freshwater ecosystem. J Pure Appl Microbiol 12(2):897–904
Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol 111:1–61
Willame R, Boutte C, Grubisic S, Wilmotte A, Komárek J, Hoffmann L (2006) Morphological and molecular characterization of planktonic cyanobacteria from Belgium and Luxembourg. J Phycol 42(6):1312–1332
Schwyn B, Neilands JB (1987) Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160:47–56
Hassan SED, Salem S, Fouda A, Awad MA, El-Gamal MS, Abdo AM (2018) New approach for antimicrobial activity and biocontrol of various pathogens by biosynthesized copper nanoparticles using endophytic actinomycetes. J Rad Res Appl Sci 11:262–270
Payne SM (1993) Iron acquisition in microbial pathogenesis. Trends Microbiol 1:66–69
Shin SH, Lim Y, Lee SE, Yang NW, Rhee JH (2001) CAS agar diffusion assay for the measurement of siderophores in biological fluids. J of Microbiol Methods 44:89–95
Pal RB, Gokarn K (2010) Siderophores and pathogenicity of microorganisms. J Biosci Tech. 1(3):127–134
Pant G, Agrawal PK (2014) Isolation and characterization of indole acetic acid producing plant growth promoting rhizobacteria from rhizospheric soil of Withania somnifera. JBSO 2(6):377–383
Miché L, Balandreau J (2001) Effects of rice seed surface sterilization with hypochlorite on inoculated Burkholderia vietnamiensis. Appl Environ Microbiol 67(7):3046–3052
Ramakrishna N, Lacey J, Smith JE (1991) Effect of surface sterilization fumigation and gamma irradiation on the microflora and germination of barley seeds. Int J Food Microbiol 13(1):47–54
Murakami C, Tanaka AR, Sato Y, Kimura Y, Morimoto K (2021) Easy detection of siderophore production in diluted growth media using an improved CAS reagent. J of Microbio Methods 189:106310. https://doi.org/10.1016/j.mimet.2021.106310
Sergeeva E, Liaimer A, Bergman B (2002) Evidence for production of the phytohormone indole-3-acetic acid by cyanobacteria. Planta 215:229–238
Babu SV, Kumar BA, Kumar NS, Karsamy PS, Varalakshmi P (2013) Indole-3-acetic acid from filamentous cyanobacteria: screening, strain identification and production. J Sci Ind Res 72:581–584
Prasanna R, Joshi M, Rana A, Nain L (2010) Modulation of IAA production in cyanobacteria by tryptophan and light. Polish J Microbio 59(2):99–105
Sorokovikova EG, Tikhonova IV, Belykh OI, Klimenkov IV, Likhoshwai EV (2008) Identification of two cyanobacterial strains isolated from the Kotel’nikovskii hot spring of the Baikal rift. Microbio 77(3):365–372
Lauterborn R (1915) Die sapropelische Lebewelt. Ein Beitrag zur Biologie des Faulschlammes natürlicher Gewässer. Verhandlungen des Naturhistorisch Medizinischen Vereins zu Heidelberg. Neue Folge 13:395–481
Khan Z, Wan Omar WM, Merican FMMS, Azizan AA, Foong CP, Convey P, Alias SA (2017) Identification and phenotypic plasticity of Pseudanabaena catenata from the Svalbard archipelago. Polish Polar Res 38(4):445–458
Acinas SG, Haverkamp T, Huisman J, Stal LJ (2009) Phenotypic and genetic diversification of Pseudanabaena spp. (cyanobacteria). ISME J 3:31–46
Komárek J (2003) Problem of the taxonomic category “species” in cyanobacteria. Algological Stud 109:281–297
Karageorgou D, Zygouri P, Tsakiridis T, Hammami MA, Chalmpes N, Subrati M, Sainis I, Spyrou K, Katapodis P, Gournis D et al (2022) Green synthesis and characterization of silver nanoparticles with high antibacterial activity using cell extracts of cyanobacterium Pseudanabaena/Limnothrix sp. Nanomaterials 12:2296. https://doi.org/10.3390/nano12132296
Veerabadhran M, Manivel N, Mohanakrishnan D, Sahal D, Muthuraman S (2014) Antiplasmodial activity of extracts of 25 cyanobacterial species from coastal regions of Tamil Nadu. Pharm Biol 52(10):1291–1301
Kulal DK, Navale DN, Zote SW, Ranade PB, Kalambate PK (2022) Cyanobacteria: as a promising candidate for nanoparticles synthesis. In: Singh P, Fillat M, Kumar A (eds) Cyanobacterial lifestyle and its applications in biotechnology. Academic Press, pp 351–360
Hamouda RA, Hussein MH, Abo-elmagd RA et al (2019) Synthesis and biological characterization of silver nanoparticles derived from the cyanobacterium Oscillatoria limnetica. Sci Rep 9:13071. https://doi.org/10.1038/s41598-019-49444-y
Singh Y, Kushal S, Sodhi RS (2020) Biogenic synthesis of silver nanoparticles using cyanobacterium Leptolyngbya sp. WUC 59 cell-free extract and their effects on bacterial growth and seed germination. Nanoscale Adv 2:3972–3982
Eltarahony M, Zaki S, Abd-El-Haleem D (2018) Concurrent synthesis of zero- and one-dimensional, spherical, rod-, needle-, and wire-shaped CuO nanoparticles by proteus mirabilis 10B. Hindawi J Nanomater. https://doi.org/10.1155/2018/1849616
Jeronsia JE, Raj DJV, Joseph LA, Rubini K, Das SJ (2016) In vitro antibacterial and anticancer activity of copper oxide nanostructures in human breast cancer Michigan cancer foundation-7 cells. J Med Sci 36(4):145–151
Arya A, Gupta K, Chundawat TS, Vaya D (2018) Biogenic synthesis of copper and silver nanoparticles using green alga Botryococcus braunii and its antimicrobial activity. Bioinorg Chem App 2018:1–9
Halder U, Roy RK, Biswas R, Khan D, Mazumder K, Bandopadhyay R (2022) Synthesis of copper oxide nanoparticles using capsular polymeric substances produced by Bacillus altitudinis and investigation of its efficacy to kill pathogenic Pseudomonas aeruginosa. Chem Eng J Adv 11:100294
Yugandhar P, Vasavi T, Uma Maheswari Devi P et al (2017) Bioinspired green synthesis of copper oxide nanoparticles from Syzygium alternifolium (Wt.) Walp: characterization and evaluation of its synergistic antimicrobial and anticancer activity. Appl Nanosci 7:417–427. https://doi.org/10.1007/s13204-017-0584-9
Pawar SM, Kim J, Inamdar AI, Woo H, Jo Y, Pawar BS, Cho S, Kim H, Im H (2016) Multi-functional reactively sputtered copper oxide electrodes for supercapacitor and electrocatalyst in direct methanol fuel cell applications. Sci Rep 6:21310
Asuquo E, Martin A, Nzerem P (2018) Evaluation of Cd (II) ion removal from aqueous solution by a low-cost adsorbent prepared from white yam (Dioscorea rotundata) waste using batch sorption. Chem Eng 3:1–36
Ijaz F, Shahid S, Khan SA, Ahmad W, Zaman S (2017) Green synthesis of copper oxide nanoparticles using Abutilon indicum leaf extract: antimicrobial, antioxidant and photocatalytic dye degradation activities. Trop J Pharm Res 16(4):743–753
Jahangirian H, Rafiee-Moghaddam R, Jahangirian N, Nikpey B, Jahangirian S, Bassous N, Saleh B, Kalantari K, Webster TJ (2020) Green Synthesis of zeolite/Fe2O3 nanocomposites: toxicity & cell proliferation assays and application as a smart iron nanofertilizer. Int J Nanomed 13(15):1005–1020
Solanki P, Bhargava A, Chhipa H, Jain N, Panwar J (2015) Nano-fertilizers and their smart delivery system. Nanotechnologies in food and agriculture. Springer, Cham, pp 81–101
Margenot AJ, Rippner DA, Dumlao MR et al (2018) Copper oxide nanoparticle effects on root growth and hydraulic conductivity of two vegetable crops. Plant Soil 431:333–345. https://doi.org/10.1007/s11104-018-3741-3
Bhattacharjee R, Kumar R, Mukerjee N et al (2022) The emergence of metal oxide nanoparticles (NPs) as a phytomedicine: a two-facet role in plant growth, nano-toxicity and anti-phyto-microbial activity. Biomed Pharma 155:113658
Acknowledgements
Authors Rajni Yadav, Manish Kumar and Rajesh Singh Tomar are thankful to Amity University Madhya Pradesh for providing continuous support during writing this research article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yadav, R., Kumar, M. & Tomar, R.S. Cyanobacteria Based Nanoformulation of Biogenic CuO Nanoparticles for Plant Growth Promotion of Rice Under Hydroponics Conditions. Curr Microbiol 81, 118 (2024). https://doi.org/10.1007/s00284-024-03619-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-024-03619-7