Abstract
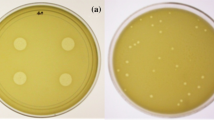
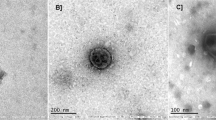
Phage therapy is a promising alternative to control bacterial diseases and the increasing problem of antibiotic resistance. In this sense, this research evaluates the viability of lyophilized vibrio phage vB_Pd_PDCC-1 using trehalose as a preservative excipient at different concentrations (4, 2, 1, and 0.5% w/v) and its potential for phage therapy application against a pathogenic bacteria Vibrio diabolicus in brine shrimp nauplii (Artemia franciscana). The lyophilized phages were stored at 4 and 23 °C and rehydrated using biological sterile saline solution to test their viability at days 1, 15, and 60 post-lyophilization. The results showed that trehalose is beneficial in maintaining the viability of post-lyophilization phages (without titer losses) at 4 °C and even at room temperature (23 °C). When lyophilized phages with 4% w/v trehalose concentration were stored at 23 °C, they had not titer losses among the trials; viability and titer concentration were maintained up to 60 days at log 7. The use of lyophilized phage PDCC-1 increased brine shrimp survival and reduced Vibrio concentrations. The present study has identified trehalose as a promising lyophilization excipient to effectively preserve lyophilized bacteriophages for biotechnological applications and long-term storage.



Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Moreno-Figueroa LD, Villarreal-Colmenares H, Naranjo-Páramo J, Vargas-Mendieta M, Mercier L, Casillas-Hernández R, Hernández-Llamas A (2019) Bioeconomic modelling of the intensive production of whiteleg shrimp (Litopenaeus vannamei) in a photo-heterotrophic hypersaline system, with minimal seawater replacement. Rev Aquac 11:685–696. https://doi.org/10.1111/raq.12252
Principi N, Silvestri E, Esposito S (2019) Advantages and limitations of bacteriophages for the treatment of bacterial infections. Front Pharmacol 10:513. https://doi.org/10.3389/fphar.2019.00513
Lomelí-Ortega CO, Martínez-Sández AJ, Barajas-Sandoval DR, Reyes AG, Magallón-Barajas F, Veyrand-Quiros B, Gannon L, Harrison C, Michniewski S, Quiroz-Guzmán E (2021) Isolation and characterization of vibriophage vB_Vc_SrVc9: an effective agent in preventing Vibrio campbellii infections in brine shrimp nauplii (Artemia franciscana). J Appl Microbiol 131(1):36–49. https://doi.org/10.1111/jam.14937
Quiroz-Guzmán E, Peña-Rodríguez A, Vázquez-Juárez R, Barajas-Sandoval DR, Balcázar JL, Martínez-Díaz SF (2018) Bacteriophage cocktails as an environmentally-friendly approach to prevent Vibrio parahaemolyticus and Vibrio harveyi infections in brine shrimp (Artemia franciscana) production. Aquaculture 492:273–279. https://doi.org/10.1016/j.aquaculture.2018.04.025
Cao Y, Zhang Y, Lan W, Sun X (2021) Characterization of vB_VpaP_MGD2, a newly isolated bacteriophage with biocontrol potential against multidrug-resistant Vibrio parahaemolyticus. Arch Virol 166:413–426. https://doi.org/10.1007/s00705-020-04887-x
Saucedo-Uriarte JA, Honorio-Javez CE, Vallenas-Sánchez YPA, Acuña-Leyva A (2020) Bacteriophages: allies to combat bacterial diseases in aquaculture. A first starting point in organic aquaculture. J Selva Andina Anim Sci 7(2):107–121
Moreno-Figueroa LD, Cab-Sulub L (2023) Bacteriófagos como biocontrol de enfermedades bacterianas en mamíferos. Therya Ixmana 2(2):47–48
Pires DP, Costa AR, Pinto G, Meneses L, Azeredo J (2020) Current challenges and future opportunities of phage therapy. FEMS Microbiol Rev 44:684–700. https://doi.org/10.1093/femsre/fuaa017
Zhang Z, Yu YX, Wang YG, Wei XX, Liao MJ, Rong XJ, Chen J (2020) Development of a new protocol for freeze-drying preservation of Pseudoalteromonas nigrifaciens and its protective effect on other marine bacteria. Electron J Biotechnol 44:1–5. https://doi.org/10.1016/j.ejbt.2019.12.006
Colom J, Cano-Sarabia M, Otero J, Cortés P, Maspoch D, Llagostera M (2015) Liposome-encapsulated bacteriophages for enhanced oral phage therapy against Salmonella spp. Appl Environ Microbiol 81:4841–4849. https://doi.org/10.1128/AEM.00812-15
Zhang Y, Peng X, Zhang H, Watts AB, Ghosh D (2018) Manufacturing and ambient stability of shelf freeze dried bacteriophage powder formulations. Int J Pharm 542:1–7. https://doi.org/10.1016/j.ijpharm.2018.02.023
Rosner D, Clark J (2021) Formulations for bacteriophage therapy and the potential uses of immobilization. Pharmaceuticals 14(359):1–19. https://doi.org/10.3390/ph14040359
Zhang M, Oldenhof H, Sydykov B, Bigalk J, Sieme H, Wolkers WF (2018) Freeze-drying of mammalian cells using trehalose: preservation of DNA integrity. Sci Rep 7:6198. https://doi.org/10.1038/s41598-017-06542-z
Chang BS, Beauvais RM, Dong A, Carpenter JF (1996) Physical factors affecting the storage stability of freeze-dried interleukin-1 receptor antagonist: glass transition and protein conformation. Arch Biochem Biophys 331:249–258. https://doi.org/10.1006/abbi.1996.0305
Manohar P, Ramesh N (2019) Improved lyophilization conditions for long-term storage of bacteriophages. Sci Rep 9:15242. https://doi.org/10.1038/s41598-019-51742-4
Hernández-Adame L, Angulo C, García-Silva I, Palestino G, Rosales-Mendoza S (2019) An overview of nanogel-based vaccines. Expert Rev Vaccines 18(9):951–968. https://doi.org/10.1080/14760584.2019.1647783
Vandenheuvel D, Singh A, Vandersteegen K, Klumpp J, Lavigne R, Mooter GVD (2013) Feasibility of spray drying bacteriophages into respirable powders to combat pulmonary bacterial infections. Eur J Pharm Biopharm 84:578–582. https://doi.org/10.1016/j.ejpb.2012.12.022
Vandenheuvel D, Meeus J, Lavigne R, Mooter GVD (2014) Instability of bacteriophages in spray-dried trehalose powders is caused by crystallization of the matrix. Int J Pharm 472:202–205. https://doi.org/10.1016/j.ijpharm.2014.06.026
Veyrand-Quirós B, Gómez-Gil B, Lomelí-Ortega CO, Escobedo-Fregoso C, Millard AD, Tovar-Ramírez D, Balcázar JL, Quiróz-Guzmán E (2020) Use of bacteriophage vB_Pd_PDCC-1 as biological control agent of Photobacterium damselae subsp. damselae during hatching of longfin yellowtail (Seriola rivoliana) eggs. J Appl Microbiol 129:1497–1510. https://doi.org/10.1111/jam.14744
Martínez-Díaz SF, Hipólito-Morales A (2013) Efficacy of phage therapy to prevent mortality during the vibriosis of brine shrimp. Aquaculture 400:120–124. https://doi.org/10.1016/j.aquaculture.2013.03.007
Cox CS, Harris WJ, Lee J (1974) Viability and electron microscope studies of phages T3 and T7 subjected to freeze-drying, freeze-thawing and aerosolization. J Gen Microbiol 81:207–215. https://doi.org/10.1099/00221287-81-1-207
Chen T, Fowler A, Toner M (2000) Supplemented phase diagram of the trehalose-water binary mixture. Cryobiology 40:277–228. https://doi.org/10.1006/cryo.2000.2244
Gutiérrez-Mosquera LF, Arias-Giraldo S, Garzón-Jiménez D, López-Velazco DM, Osorio-Alturo A (2014) Transición vítrea en alimentos: sistemas binarios agua-carbohidratos. Vector 9:21–28
Ly A, Carrigy NB, Wang H, Harrison M, Sauvageau D, Martin AR, Vehring R, Finlay WH (2019) Atmospheric spray freeze drying of sugar solution with phage D29. Front Microbiol 10:488. https://doi.org/10.3389/fmicb.2019.00488
Petsong K, Benjakul S, Vongkamjan K (2021) Optimization of wall material for phage encapsulation via freeze-drying and antimicrobial efficacy of microencapsulated phage against Salmonella. J Food Sci Technol 58(5):1937–1946. https://doi.org/10.1007/s13197-020-04705-x
Russ N, Zielbauer BI, Vilgis TA (2014) Impact of sucrose and trehalose on different agarose-hydrocolloid systems. Food Hidrocoll 41:44–52. https://doi.org/10.1016/j.foodhyd.2014.03.020
Malferrari M, Nalepa A, Venturolo G, Francia F, Lubitz W, Mobius K, Savitsky A (2014) Structural and dynamical characteristics of trehalose and sucrose matrices at different hydration levels as probed by FTIR and high-field EPR. Phys Chem Chem Phys 16:9831–9848. https://doi.org/10.1039/C3CP54043J
Sloan AWN, Santana-Pereira ALR, Goswami J, Liles MR, Davis VA (2017) Single-walled carbon nanotube dispersion in tryptic soy broth. ACS Macro Lett 6(11):1228–1231. https://doi.org/10.1021/acsmacrolett.7b00656
Márquez MJ, Romani D, Díaz SB, Brandán SA (2018) Structural and vibrational characterization of anhydrous and dihydrated species of trehalose based on the FTIR and FTRaman spectra and DFT calculations. J King Saud Univ Sci 30(2):229–249. https://doi.org/10.1016/j.jksus.2017.01.009
Pérez-Esteban P, Jenkings ATA, Arnot TC (2016) Elucidation of the mechanisms of action of bacteriophage K/nano-emulsion formulations against S. aureus via measurement of particle size and zeta potential. Colloids Surf B 139:87–94. https://doi.org/10.1016/j.colsurfb.2015.11.030
Wannet WJ, Op den Camp HJ, Wisselink HW, Van der Drift C, Van Griensven LJ, Vogels GD (1998) Purification and characterization of trehalose phosphorylase from the commercial mushroom Agaricus bisporus. Biochim Biophys Acta 1425:177–188. https://doi.org/10.1016/s0304-4165(98)00066-x
Bosch S, de Beaurepaire L, Allard M, Mosser M, Heichette C, Chrétien D, Jegou D, Bach JM (2016) Trehalose prevents aggregation of exosomes and cryodamage. Sci Rep 6:36162. https://doi.org/10.1038/srep36162
Mensink MA, Frijlink HW, Hinrichs WL, Voort VD, Maarschalk K (2017) How sugars protect proteins in the solid state and during drying: mechanisms of stabilization in relation to stress conditions. Eur J Pharm Biopharm 114:288–295. https://doi.org/10.1016/j.ejpb.2017.01.024
Ávila-Rincón L, Naranjo-Vasco JM, Higuita-Vásquez JC (2015) Viabilidad de levaduras y bacterias conservadas por liofilización: efecto de agentes lioprotectores. Vector 10:7–13
Lee-Shuan L, Kayasuga-Kariya Y, Nakamura S, Nobuyuki S, Sakai T, Fujisawa A, Akagi Y, Suzuki S, Chung U, Sasaki N, Mochizuki M (2016) Co-lyophilized aspirin with trehalose causes less injury to human gastric cells and gastric mucosa of rats. Dig Dis Sci 61(8):2242–2251. https://doi.org/10.1007/s10620-016-4209-z
Li L, Wang P, Xu Y, Wu X, Liu X (2022) Effect of trehalose on the physicochemical properties of freeze-dried powder of royal jelly of northeastern black bee. Coatings 12:173. https://doi.org/10.3390/coatings12020173
Jain KN, Roy I (2010) Trehalose and protein stability. In: Current protocols in protein science. Wiley Interscience, New York
Merabishvili M, Vervaet C, Pirnay JP, De Vos D, Verbeken G, Mast J, Chanishvili N, Vaneechoutte M (2013) Stability of Staphylococcus aureus phage ISP after freeze-drying (lyophilization). PLoS ONE 8(7):e68797. https://doi.org/10.1371/journal.pone.0068797
Davies JD, Kelly MJ (1969) The preservation of bacteriophage H1 of Corynebacterium ulcerans U103 by freeze-drying. J Hyg 67:573–583. https://doi.org/10.1017/s0022172400042030
Carne HR, Greaves RI (1974) Preservation of corynebacteriophages by freeze-drying. J Hyg 72:467–470. https://doi.org/10.1017/s0022172400023706
Puapermpoonsiri U, Spencer J, Van Der Walle CF (2009) A freeze-dried formulation of bacteriophage encapsulated in biodegradable microspheres. Eur J Pharm Biopharm 72:26–33. https://doi.org/10.1016/j.ejpb.2008.12.001
Clark WA (1962) Comparison of several methods for preserving bacteriophages. J Appl Microbiol 10:466–471. https://doi.org/10.1128/am.10.5.466-471.1962
Zierdt CH (1988) Stabilities of lyophilized Staphylococcus aureus typing bacteriophages. Appl Environ Microbiol 54(10):2590. https://doi.org/10.1128/aem.54.10.2590-.1988
Ackermann HW, Tremblay D, Moineau S (2004) Long-term bacteriophage preservation. World Fed Cult Collect Newsl 38:35–40
Cleland JL, Lam X, Kendrick B, Yang J, Yang TH, Overcashier D, Brooks D, Hsu C, Carpenter JF (2001) A specific molar ratio of stabilizer to protein is required for storage stability of a lyophilized monoclonal antibody. J Pharm Sci 90:310–321. https://doi.org/10.1002/1520-6017(200103)90:3%3c310::aid-jps6%3e3.0.co;2-r
Hoe S, Semler DD, Goudie AD, Lynch KH, Matinkhoo S, Finaly WH (2013) Respirable bacteriophages for the treatment of bacterial lung infections. J Aerosol Med Pulm 26:317–335. https://doi.org/10.1089/jamp.2012.1001
Moreno-Figueroa LD, Naranjo-Páramo J, Hernández-Llamas A, Vargas-Mendieta M, Hernández-Gurrola JA, Villarreal-Colmenares H (2018) Performance of a photo-heterotrophic, hypersaline system for intensive cultivation of white leg shrimp (Litopenaeus vannamei) with minimal water replacement in lined ponds using a stochastic approach. Aquac Res 49:57–67. https://doi.org/10.1111/are.13432
Lomelí-Ortega CO, Martínez-Díaz SF (2014) Phage therapy against Vibrio parahaemolyticus infection in the whiteleg shrimp (Litopenaeus vannamei) larvae. Aquaculture 434:208–211. https://doi.org/10.1016/j.aquaculture.2014.08.018
Quiroz-Guzmán E, Balcázar JL, Vázquez-Juárez R, Cruz-Villacorta AA, Martínez-Díaz SF (2013) Proliferation, colonization, and detrimental effects of Vibrio parahaemolyticus and Vibrio harveyi during brine shrimp hatching. Aquaculture 406–407:85–90. https://doi.org/10.1016/j.aquaculture.2013.03.008
Acknowledgements
The authors acknowledge the technical assistance of Norma Angélica Ochoa Álvarez, José Manuel Melero Astorga, and María Sofía Ramos Galván at CIBNOR. Dr. Luis Daniel Moreno-Figueroa (CVU: 336817) is a recipient of a postdoctoral fellowship from the Consejo Nacional de Ciencia y Tecnología of Mexico (CONACYT) and Diana Fischer for English edition.
Funding
This work was funded by the Project “Infraestructura CONACYT” No. 316934 awarded to Dr. Luis Hernández-Adame.
Author information
Authors and Affiliations
Contributions
LDMF was contributed to conceptualization, data curation, formal analysis, methodology, and writing of the original draft. LHA contributed to conceptualization, data curation, formal analysis, funding acquisition, project administration, supervision, and writing, reviewing, and editing of the manuscript. GP contributed to data curation, validation, and formal analysis. CACC contributed to data curation, validation, and formal analysis. EQG contributed to data curation, validation, formal analysis, and writing, reviewing, and editing of the manuscript. DTR contributed to data curation, validation, formal analysis, and writing, reviewing, and editing of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest relevant to this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Moreno-Figueroa, L.D., Quiroz-Guzmán, E., Tovar-Ramírez, D. et al. Use of Trehalose as an Additive to Bacteriophage Vb_Pd_PDCC-1: Long-Term Preservation Analysis and Its Biocontrol Against Vibrio diabolicus Infection. Curr Microbiol 80, 372 (2023). https://doi.org/10.1007/s00284-023-03487-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-023-03487-7