Abstract
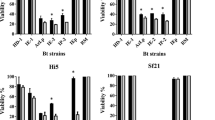
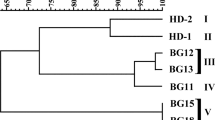
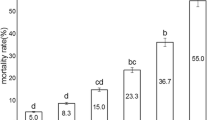
Bacillus thuringiensis (Bt) is the most used technology for biological control of insect pathogens worldwide. In order to select new Bt candidates challenging the emergence of insect’s resistance, a mass bioassay and molecular screening was performed on an autochthonous collection. Toxicity assays against neonate larvae of three lepidopteran species (Mamestra brassicae, Grapholita molesta, and Spodoptera exigua) were conducted using spore–crystal mixtures and supernatant cultures of 49 Bt isolates harboring at least one gene coding for a lepidopteran-specific insecticidal protein. A threshold of 30% of “functional mortality” was used to discriminate between “nontoxic” and “toxic” isolates. The toxicity of many Bt isolates competed with that of Btk-HD1. However, only three of them (Bl4NA, Bl5NA, and Bl9NA) showed high toxicity in both spore–crystal mixtures and supernatant cultures against the three lepidopteran species. The Bt isolates Bl4NA and Bl9NA express a protein of 130 kDa whereas the Bt isolate Bl5NA expresses a protein of 65–70 kDa. The LC–MS/MS results indicate that the major peptides in the 130 kDa band of Bl9NA were Cry1Da, Cry1Ca, Cry1Ab, and Cry1Aa, and those in the 70 kDa band of Bl5NA were Cry1Aa and Cry1Ca. The evaluation of the protein content of the supernatants by comparison to Btk-HD1 indicates the overproduction of Vip3 proteins in these strains (most likely Vip3Aa in Bl4NA and Bl9NA and Vip3Ca in Bl5NA). In addition, these three Bt strains do not produce β-exotoxins. Based on our results, the three selected strains could be considered promising candidates to be used in insect pest control.




Similar content being viewed by others
References
Schnepf E, Crickmore N, Van Rie J et al (1998) Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol Mol Biol Rev 62:775–806. https://doi.org/10.1128/mmbr.62.3.775-806.1998
Palma L, Muñoz D, Berry C et al (2014) Bacillus thuringiensis toxins: an overview of their biocidal activity. Toxins 6:3296–3325. https://doi.org/10.3390/toxins6123296
Sanchis V, Bourguet D (2008) Bacillus thuringiensis: applications in agriculture and insect resistance management. A review. In: Lichtfouse E, Navarrete M, Debaeke P, Véronique S, Alberola C (eds) Sustainable agriculture. Springer, Dordrecht, pp 243–255. https://doi.org/10.1007/978-90-481-2666-8_16
Höfte H, Whiteley HR (1989) Insecticidal crystal proteins of Bacilllus thuringiensis. Microbiol Rev 53(2):242–255
Tabashnik BE, Van RJBJ, Carrière Y (2009) Field-evolved insect resistance to Bt Crops : Definition, theory, and data. J Econ Entomol 102:2011–2025. https://doi.org/10.1603/029.102.0601
Bravo A, Likitvivatanavong S, Gill SS, Soberón M (2011) Bacillus thuringiensis: a story of a successful bioinsecticide. Insect Biochem Mol Biol 41:423–431. https://doi.org/10.1016/j.ibmb.2011.02.006
Xu C, Wang BC, Yu Z, Sun M (2014) Structural insights into Bacillus thuringiensis Cry, Cyt and parasporin toxins. Toxins 6:2732–2770. https://doi.org/10.3390/toxins6092732
Jurat-Fuentes JL, Crickmore N (2017) Specificity determinants for Cry insecticidal proteins: insights from their mode of action. J Invertebr Pathol 142:5–10. https://doi.org/10.1016/j.jip.2016.07.018
Estruch JJ, Warren GW, Mullins MA et al (1996) Vip3A, a novel Bacillus thuringiensis vegetative insecticidal protein with a wide spectrum of activities against lepidopteran insects. Proc Natl Acad Sci 93:5389–5394. https://doi.org/10.1073/pnas.93.11.5389
Ruiz de Escudero I, Banyuls N, Bel Y et al (2014) A screening of five Bacillus thuringiensis Vip3A proteins for their activity against lepidopteran pests. J Invertebr Pathol 117:51–55. https://doi.org/10.1016/j.jip.2014.01.006
Sena JAD, Hernández-Rodríguez CS, Ferre J (2009) Interaction of Bacillus thuringiensis Cry1 and Vip3A Proteins with Spodoptera frugiperda midgut binding sites. Appl Environ Microbiol 75:2236–2237. https://doi.org/10.1128/AEM.02342-08
Chakroun M, Banyuls N, Bel Y, Escriche B, Ferré J (2016) Bacterial Vegetative Insecticidal Proteins (Vip) from entomopathogenic bacteria. Microbiol Mol Biol 80:329–350. https://doi.org/10.1128/MMBR.00060-15.Address
Hernández-Rodríguez CS, Boets A, Van Rie J, Ferré J (2009) Screening and identification of vip genes in Bacillus thuringiensis strains. J Appl Microbiol 107:219–225. https://doi.org/10.1111/j.1365-2672.2009.04199.x
Djenane Z, Nateche F, Amziane M et al (2017) Assessment of the antimicrobial activity and the entomocidal potential of Bacillus thuringiensis isolates from Algeria. Toxins 9(4):139. https://doi.org/10.3390/toxins9040139
World Health Organization (1999) Guidelines Specification for bacterial larvicides for public health use, WHO/CDS/CPC/WHOPES/99.2 report of the WHO informal consultation. World Health Organization, Geneva
Guennelon G, Audemard H, Fremond J-C, El Idrissi Ammari MA (1981) Progrès réalisés dans l’élevage permanent du Carpocapse (Laspeyresia pomonella L.) sur milieu artificiel. Agronomie 1:59–64. https://doi.org/10.1051/agro:19810108
Poitout S, Bues R (1974) Élevage des chenilles de vinghuit espèces de lépidoptères Noctuidae et de deux espèces d’Arctiidae sur milieu artificiei simple. Particularités de l’élevage selon les espèces. Ann Ecol Anim 6:431–441. https://doi.org/10.1111/j.1570-7458.1972.tb00219.x
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685. https://doi.org/10.1038/227680a0
Gomis-Cebolla J, Scaramal Ricietto AP, Ferré J (2018) A genomic and proteomic approach to identify and quantify the expressed Bacillus thuringiensis. Toxins 10(5):1–18. https://doi.org/10.3390/toxins10050193
Şahin B, Gomis-Cebolla J, Günes H, Ferré J (2018) Characterization of Bacillus thuringiensis isolates by their insecticidal activity and their production of Cry and Vip3 proteins. PLoS ONE 13:1–18. https://doi.org/10.1371/journal.pone.0206813
Hernández CS, Martínez C, Porcar M et al (2003) Correlation between serovars of Bacillus thuringiensis and type I beta-exotoxin production. J Invertebr Pathol 82:57–62. https://doi.org/10.1016/s0022-2011(02)00199-4
Khorramnejad A, Talaei-Hassanloui R, Hosseininaveh V et al (2018) Characterization of new Bacillus thuringiensis strains from Iran, based on cytocidal and insecticidal activity, proteomic analysis and gene content. Biocontrol 63:807–818. https://doi.org/10.1007/s10526-018-9901-9
Gould F, Anderson A, Reynolds A et al (1995) Selection and genetic analysis of a Heliothis virescens (Lepidoptera: Noctuidae) strain with high levels of resistance to Bacillus thuringiensis toxins. J Econ Entomol 88:1545–1559. https://doi.org/10.1093/jee/88.6.1545
Palma L, Hernández-Rodríguez CS, Maeztu M et al (2012) Vip3C, a novel class of vegetative insecticidal proteins from Bacillus thuringiensis. Appl Environ Microbiol 78:7163–7165. https://doi.org/10.1128/AEM.01360-12
Gomis-cebolla J, Ruiz de Escudero I, Vera-velasco NM et al (2016) Insecticidal spectrum and mode of action of the Bacillus thuringiensis Vip3Ca insecticidal protein. J Invertebr Pathol 142:60–67. https://doi.org/10.1016/j.jip.2016.10.001
Lemes AR, Davolos CC, Legori PC et al (2014) Synergism and antagonism between Bacillus thuringiensis Vip3A and Cry1 proteins in Heliothis virescens, Diatraea saccharalis and Spodoptera frugiperda. PLoS ONE 9:e107196. https://doi.org/10.1371/journal.pone.0107196
Wang Z, Fang L, Zhou Z et al (2018) Specific binding between Bacillus thuringiensis Cry9Aa and Vip3Aa toxins synergizes their toxicity against Asiatic rice borer (Chilo suppressalis). J Biol Chem 293:11447–11458. https://doi.org/10.1074/jbc.RA118.003490
Acknowledgements
We thank Dr. Yolanda Bel for her valuable comments related to toxicity bioassays, Dr. Joaquín Gomis-Cebolla for his help in the analysis of the proteomics data, and Dr. Patricia Hernández-Martínez and Daniel Pinos for their help in SDS-PAGE and Dot-Blot assays. We thank Rosa Maria González-Martínez for her support in laboratory assistance and Oscar Marín-Vázquez for his help with insect rearing. This work was supported by PNE research grant from the Algerian Ministry of Higher Education and Scientific Research (n° 100/PNE/ENS./ESPAGNE/2015–2016), by the Spanish Ministry of Science and Innovation (Grant Ref. AGL2015-70584-C2-1-R), by a Grant of the Generalitat Valenciana (GVPROMETEOII-2015-001) and by European FEDER funds. The proteomic analysis was performed in the proteomics facility of SCSIE University of Valencia that belongs to ProteoRed, PRB2-ISCIII, supported by Grant PT13/0001 of the PE I+D+i 2013–2016, funded by ISCIII and FEDERPT13/0001.
Author information
Authors and Affiliations
Contributions
JF and FN conceived and designed the experiments; ZD and ML-B performed the insecticidal and molecular characterization of the strains; ZD, ML-B, and JF analyzed the results and wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical Approval
Animal ethics approval is not required for studies using Mamestra brassicae, Grapholita molesta, and Spodoptera exigua invertebrates.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Djenane, Z., Lázaro-Berenguer, M., Nateche, F. et al. Evaluation of the Toxicity of Supernatant Cultures and Spore–Crystal Mixtures of Bacillus thuringiensis Strains Isolated from Algeria. Curr Microbiol 77, 2904–2914 (2020). https://doi.org/10.1007/s00284-020-02110-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-020-02110-3