Abstract
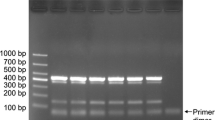
Ralstonia solanacearum (Smith) Yabuuchi et al. and Erwinia carotovora subsp. carotovora (Jones) Bergey et al. (Pectobacterium carotovorum subsp. carotovorum) are the two major bacterial pathogens of potato causing brown rot (wilt) and soft rot diseases, respectively, in the field and during storage. Reliable and early detection of these pathogens are keys to avoid occurrence of these diseases in potato crops and reduce yield loss. In the present study, multiplex polymerase chain reaction (PCR) protocol was developed for simultaneous detection of R. solanacearum and E. carotovora subsp. carotovora from potato tubers. A set of oligos targeting the pectatelyase (pel) gene of E. carotovora subsp. carotovora and the universal primers based on 16S r RNA gene of R. solanacearum were used. The standardized multiplex PCR protocol could detect R. solanacearum and E. carotovora subsp. carotovora up to 0.01 and 1.0 ng of genomic DNA, respectively. The protocol was further validated on 96 stored potato tuber samples, collected from different potato-growing states of India, viz. Uttarakhand, Odisha, Meghalaya and Delhi. 53.1 % tuber samples were positive for R. solanacearum, and 15.1 % of samples were positive for E. carotovora subsp. carotovora, and both the pathogens were positive in 26.0 % samples when BIO-PCR was used. This method offers sensitive, specific, reliable and fast detection of two major bacterial pathogens from potato tubers simultaneously, particularly pathogen-free seed certification in large scale.






Similar content being viewed by others
References
Agrios GN (2006) Bacterial soft rots 5th Edn Academic Press San Diego
Alvarez AM (2004) Integrated approaches for detection of plant pathogenic bacteria and diagnosis of bacterial disease. Annu Rev Phytopathol 42:339–366
Bergey DH, Harrison FC, Bread RS, Hammer BW, Huntoon FM (1939) Bergey’s manual of determinative bacteriology. Williams and Wilkins, Baltimore
Ciampi L Sequeira L French ER (1981) Pseudomonas solanacearum distribution in potato plants and the establishment of latent infections. In: C Lozano (Ed) Proceedings of the 5th international conference on plant pathogenic Bacteria cali, Colombia Centro Internacional de Agricultura Tropical (pp 148–161)
Czajkowski R, Perombelon MCM, Van Veen JA, Van der Wolf JM (2012) Control of blackleg and tuber soft rot of potato caused by Pectobacterium and Dickeya species: a review. Plant Pathol 60:999–1013
Denny TP (2006) Plant pathogenic Ralstonia species. In: Gnanamanickam SS (ed) Plant associated bacteria. Springer, Dordtrecht, pp 573–644
Elphinstone JG (2005) The current bacterial wilt situation: a global overview. In: Allen C, Prior P, Hayward AC (eds) Bacterial wilt disease and the Ralstonia solanacearum species complex. APS, New York, pp 9–28
Grimberg J, Maguire S, Belluscio L (1989) A simple method for the preparation of plasmid and chromosomal E. coli DNA. Nucleic Acid Res 17:8893
Hayward AC (1991) Biology and epidemiology of baterial wilt caused by Pseudomonas solanacearum. Annu Rev Phytopathol 29:65–87. doi:10.1146/annurevpy29090191000433
Hélias V, Andrivon D, Jouan B (2000) Internal colonization pathways of potato plants by Erwinia carotovora subsp. atroseptica. Plant Pathol 49:33–42
Helias V, Hamon P, Huchet E, van der Wolf JM, Andrivon D (2011) Two new effective semiselective crystal violet pectate media for isolation of Pectobacterium and Dickeya. Plant Pathol 61:339–345
Hyman LJ, Sullivan L, Toth IK, Perombelon MCM (2001) Modified crystal violet pectate medium (CVP) based on a new polypectate source (Slendid) for the detection and isolation of soft rot erwinias. Potato Res 44:265–270
Ito S, Ushijima Y, Fujii T, Tanaka S, Kameya-Iwaki M, Yoshiwara S, Kishi F (1998) Detection of viable cells of Ralstonia solanacearum in soil using a semi-selective medium and a PCR technique. J Phyto pathol 146:379–384
Janse JD, Wenneker M (2002) Possibilities of avoidance and control of bacterial plant diseases when using pathogen-tested (certified) or -treated planting material. Plant Pathol 51:523–536
Larka BS (2004) Integrated approach for the management of soft rot (Pectobacterium carotovorum subsp. carotovorum) of radish (Raphanus sativus) seed crop. Haryana J Agron 20:128–129
Lopez M, Bertolini E, Olmos A, Caruso P, Gorris M, Llop P, Penyalver R, Cambra M (2003) Innovative tools for detection of plant pathogenic viruses and bacteria. Inter Microbiol 6:233–243
Markoulatos P, Siafakas N, Moncany M (2002) Multiplex polymerase chain reaction: a practical approach. J Clin Lab Anal 16:47–51
Mikicinski A, Sobiczewski P, Puławska J, Treder J (2010) Involvement of Paenibacillus polymyxa in the etiology of bacterial soft rot of Calla Lily. J Plant Pathol 92:375–380
Mikicinski A, Sobiczewski P, Sulikowska M, Puławska J, Treder J (2010) Pectolytic bacteria associated with soft rot of Calla Lily (Zantedeschia spp.) tubers. J Phyto pathol 158:201–209
Murray MG, Thompson WF (1980) Rapid isolation of high molecular weight DNA. Nucl Acids Res 8:4321–4325
Opina N, Tavner F, Hollway G, Wang JF, Li TH, Maghirang R, Fegan M, Hayward AC, Krishnapillai V, Hong WF, Holloway BW, Timmis JN (1997) A novel method for development of species and strain specific DNA probes and PCR primer for identifying Burkholderia solanacearum. Asia Pac J Mol Bio 5:19–30
Ozakman M, Schaad NW (2003) A real-time BIO-PCR assay for detection of Ralstonia solanacearum race 3, biovar 2, in asymptomatic potato tubers. Can J Plant Pathol 25:232–239
Potrykus M, Sledz W, Golanowska M, Slawiak M, Binek A, Motyka A, Zoledowska S, Lojkowska EC (2014) Simultaneous detection of major blackleg and sot rot bacterial pathogens in potato by multiplex polymerase chain reaction. Ann Appl Biol 165:474–487
Pradhanang PM, Elphinstone JG, Fox RTV (2000) Sensitive detection of Ralstonia solanacearum in soil: a comparison of different detection techniques. Plant Pathol 49:414–422
Schaad NW, Jones JB, Chun W (2001) Laboratory guide for identification of plant pathogenic bacteria. APS, New York, pp 154–174
Singh D, Dhar S (2011) Bio-PCR based diagnosis of Xanthomonas campestris pathovars in black rot infected leaves of crucifers. Indian Phytopathol 64(1):7–11
Dinesh Singh, Shweta Sinha, Yadav DK (2014) Detection of Ralstonia solanacearum from asymptomatic tomato, Irrigation water and soil through non-selective enrichment medium with hrp Gene-based Bio-PCR. Curr Microbiol 69:127–134
Somani AK, Chakrabarti SK, Pandey SK (2010) Spread of bacterial wilt and brown rot of potato in Indore region of Madhya Pradesh. CPRI News Lett 42:16–17
Stulberg MJ, Shao J, Huang Q (2015) A multiplex PCR assay to detect and differentiate seletcted agent strains of Ralstonia solanacearum. Plant Dis 99:333–341
Ward E, Foster JS, Fraaije BA, McCartney HA (2004) Plant pathogen diagnostic: immunological and nucleic acid-based approaches. Ann Appl Biol 145:1–16
Zielke R, Naumann K (1984) Untersuchungenzur Erfassung des latenten Befallsstadiums von Corynebacterium sepedonicum (Spieck. etKotth.) Skapt. etBurkh. imKartoffelgewebe. Zentralblattfürmikrobiologie 139(4):267–280
Acknowledgments
The authors are thankful to ICAR for financial assistance. The authors are also thankful to the Head, the Division of Plant Pathology, IARI, New Delhi for providing research facilities, planning and encouragement to execute the research work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ranjan, R.K., Singh, D. & Baranwal, V.K. Simultaneous Detection of Brown Rot- and Soft Rot-Causing Bacterial Pathogens from Potato Tubers Through Multiplex PCR. Curr Microbiol 73, 652–659 (2016). https://doi.org/10.1007/s00284-016-1110-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-016-1110-0