Abstract
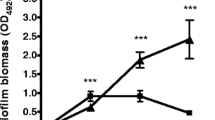
Many diseases caused by Staphylococcus aureus are associated with biofilm formation. However, the ability of S. aureus isolates from skin and soft tissue infections to form biofilms has not yet been investigated. We tested 160 isolates from patients with various skin infections for biofilm-forming capacity in different growth media. All the isolates formed biofilms, the extent of which depended on the type of growth medium. The thickest biofilms were formed when both plasma and glucose were present in the broth; in this case, S. aureus incorporated host fibrin into the biofilm’s matrix. There were no differences in the biofilm formation between isolates from different types of skin infections, except for a particularly good biofilm formation by isolates from diabetic wounds and a weaker biofilm formation by isolates from impetigo. In conclusion, biofilm formation is a universal behavior of S. aureus isolates from skin infections. In some cases, such as in diabetic wounds, a particularly strong biofilm formation most likely contributes to the chronic and recurrent character of the infection. Additionally, as S. aureus apparently uses host fibrin as part of the biofilm structure, we suggest that plasma should be included more frequently in in vitro biofilm studies.


Similar content being viewed by others
References
Akiyama H, Hamada T, Huh WK, Yamasaki O, Oono T, Fujimoto W, Iwatsuki K (2003) Confocal laser scanning microscopic observation of glycocalyx production by Staphylococcus aureus in skin lesions of bullous impetigo, atopic dermatitis and pemphigus foliaceus. Br J Dermatol 148:526–532
Akiyama H, Huh WK, Fujii K, Yamasaki O, Oono T, Iwatsuki K (2002) Confocal laser microscopic observation of glycocalyx production by Staphylococcus aureus in vitro. J Dermatol Sci 29:54–61
Akiyama H, Huh WK, Yamasaki O, Oono T, Iwatsuki K (2002) Confocal laser scanning microscopic observation of glycocalyx production by Staphylococcus aureus in mouse skin: does S. aureus generally produce a biofilm on damaged skin? Br J Dermatol 147:879–885
Akiyama H, Ueda M, Kanzaki H, Tada J, Arata J (1997) Biofilm formation of Staphylococcus aureus strains isolated from impetigo and furuncle: role of fibrinogen and fibrin. J Dermatol Sci 16:2–10
Ando E, Monden K, Mitsuhata R, Kariyama R, Kumon H (2004) Biofilm formation among methicillin-resistant Staphylococcus aureus isolates from patients with urinary tract infection. Acta Med Okayama 58:207–214
Archer NK, Mazaitis MJ, Costerton JW, Leid JG, Powers ME, Shirtliff ME (2011) Staphylococcus aureus biofilms: properties, regulation, and roles in human disease. Virulence 2:445–459
Chen P, Abercrombie JJ, Jeffrey NR, Leung KP (2012) An improved medium for growing Staphylococcus aureus biofilm. J Microbiol Methods 90:115–118
Croes S, Deurenberg RH, Boumans ML, Beisser PS, Neef C, Stobberingh EE (2009) Staphylococcus aureus biofilm formation at the physiologic glucose concentration depends on the S. aureus lineage. BMC Microbiol 9:229
Davis SC, Martinez L, Kirsner R (2006) The diabetic foot: the importance of biofilms and wound bed preparation. Curr Diab Rep 6:439–445
Davis SC, Ricotti C, Cazzaniga A, Welsh E, Eaglstein WH, Mertz PM (2008) Microscopic and physiologic evidence for biofilm-associated wound colonization in vivo. Wound Repair Regen 16:23–29
Ferreira FA, Souza RR, Bonelli RR, Americo MA, Fracalanzza SE, Figueiredo AM (2012) Comparison of in vitro and in vivo systems to study ica-independent Staphylococcus aureus biofilms. J Microbiol Methods 88:393–398
Foster TJ, Geoghegan JA, Ganesh VK, Höök M (2014) Adhesion, invasion and evasion: the many functions of the surface proteins of Staphylococcus aureus. Nat Rev Microbiol 12:49–62
Gurjala AN, Geringer MR, Seth AK, Hong SJ, Smeltzer MS, Galiano RD, Leung KP, Mustoe TA (2011) Development of a novel, highly quantitative in vivo model for the study of biofilm-impaired cutaneous wound healing. Wound Repair Regen 19:400–410
Han A, Zenilman JM, Melendez JH, Shirtliff ME, Agostinho A, James G, Stewart PS, Mongodin EF, Rao D, Rickard AH, Lazarus GS (2011) The importance of a multifaceted approach to characterizing the microbial flora of chronic wounds. Wound Repair Regen 19:532–541
James GA, Swogger E, Wolcott R, Pulcini E, Secor P, Sestrich J, Costerton JW, Stewart PS (2008) Biofilms in chronic wounds. Wound Repair Regen 16:37–44
Katsuyama M, Ichikawa H, Ogawa S, Ikezawa Z (2005) A novel method to control the balance of skin microflora. Part 1. Attack on biofilm of Staphylococcus aureus without antibiotics. J Dermatol Sci 38:197–205
Kiedrowski MR, Horswill AR (2011) New approaches for treating staphylococcal biofilm infections. Ann N Y Acad Sci 1241:104–121
Kwiecinski J, Jacobsson G, Karlsson M, Zhu X, Wang W, Bremell T, Josefsson E, Jin T (2013) Staphylokinase promotes the establishment of Staphylococcus aureus skin infections while decreasing disease severity. J Infect Dis 208:990–999
Lovati AB, Drago L, Monti L, De Vecchi E, Previdi S, Banfi G, Romano CL (2013) Diabetic mouse model of orthopaedic implant-related Staphylococcus aureus infection. PLoS ONE 8:e67628
Nemoto K, Hirota K, Ono T, Murakami K, Nagao D, Miyake Y (2000) Effect of Varidase (streptokinase) on biofilm formed by Staphylococcus aureus. Chemotherapy 46:111–115
O’Neill E, Pozzi C, Houston P, Smyth D, Humphreys H, Robinson DA, O’Gara JP (2007) Association between methicillin susceptibility and biofilm regulation in Staphylococcus aureus isolates from device-related infections. J Clin Microbiol 45:1379–1388
Podbielska A, Galkowska H, Stelmach E, Mlynarczyk G, Olszewski WL (2010) Slime production by Staphylococcus aureus and Staphylococcus epidermidis strains isolated from patients with diabetic foot ulcers. Arch Immunol Ther Exp (Warsz) 58:321–324
Pulcrano G, Vollaro A, Rossano F, Catania MR (2013) Molecular and phenotypic characterization of methicillin-resistant Staphylococcus aureus from surgical site infections. Surg Infect (Larchmt) 14:196–202
Roche ED, Renick PJ, Tetens SP, Ramsay SJ, Daniels EQ, Carson DL (2012) Increasing the presence of biofilm and healing delay in a porcine model of MRSA-infected wounds. Wound Repair Regen 20:537–543
Schierle CF, De la Garza M, Mustoe TA, Galiano RD (2009) Staphylococcal biofilms impair wound healing by delaying reepithelialization in a murine cutaneous wound model. Wound Repair Regen 17:354–359
Seth AK, Geringer MR, Galiano RD, Leung KP, Mustoe TA, Hong SJ (2012) Quantitative comparison and analysis of species-specific wound biofilm virulence using an in vivo, rabbit-ear model. J Am Coll Surg 215:388–399
Shin K, Yun Y, Yi S, Lee HG, Cho JC, Suh KD, Lee J, Park J (2013) Biofilm-forming ability of Staphylococcus aureus strains isolated from human skin. J Dermatol Sci 71:130–137
Sjolund M, Kahlmeter G (2008) Staphylococci in primary skin and soft tissue infections in a Swedish county. Scand J Infect Dis 40:894–898
Smith K, Perez A, Ramage G, Lappin D, Gemmell CG, Lang S (2008) Biofilm formation by Scottish clinical isolates of Staphylococcus aureus. J Med Microbiol 57:1018–1023
Stepanovic S, Vukovic D, Dakic I, Savic B, Svabic-Vlahovic M (2000) A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J Microbiol Methods 40:175–179
Sun Y, Dowd SE, Smith E, Rhoads DD, Wolcott RD (2008) In vitro multispecies Lubbock chronic wound biofilm model. Wound Repair Regen 16:805–813
Szczuka E, Urbanska K, Pietryka M, Kaznowski A (2013) Biofilm density and detection of biofilm-producing genes in methicillin-resistant Staphylococcus aureus strains. Folia Microbiol (Praha) 58:47–52
Vanassche T, Kauskot A, Verhaegen J, Peetermans WE, van Ryn J, Schneewind O, Hoylaerts MF, Verhamme P (2012) Fibrin formation by staphylothrombin facilitates Staphylococcus aureus-induced platelet aggregation. Thromb Haemost 107:1107–1121
Vanassche T, Peetermans M, Van Aelst LN, Peetermans WE, Verhaegen J, Missiakas DM, Schneewind O, Hoylaerts MF, Verhamme P (2013) The role of staphylothrombin-mediated fibrin deposition in catheter-related Staphylococcus aureus infections. J Infect Dis 208:92
Walker JN, Horswill AR (2012) A coverslip-based technique for evaluating Staphylococcus aureus biofilm formation on human plasma. Front Cell Infect Microbiol 2:39
Acknowledgments
We are grateful to Maria Karlsson, whose research was essential for the creation of the isolate collection. This study was supported by the Swedish Medical Research Council, the Swedish agreement concerning research and education of doctors, the Göteborg Medical Society, the Swedish Medical Society, the Rune and Ulla Amlövs Foundation, the Tore Nilsons Foundation, the Wilhelm and Martina Lundgren Foundation, the Göteborg Association against Rheumatism, the Stiftelsen Clas Groschinskys Minnesfond, and the University of Gothenburg.
Conflict of interest
The authors declare no competing interests.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kwiecinski, J., Kahlmeter, G. & Jin, T. Biofilm Formation by Staphylococcus aureus Isolates from Skin and Soft Tissue Infections. Curr Microbiol 70, 698–703 (2015). https://doi.org/10.1007/s00284-014-0770-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-014-0770-x