Abstract
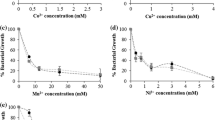
The latency global regulator DosR regulon of Mycobacterium tuberculosis, which is stimulated by hypoxia, comprises approximately fifty genes including ctpF (Rv1997), which encodes a putative alkali/alkaline earth ion transporter of the plasma membrane. In this work, the influence of hypoxia and M. tuberculosis DosR on the ATPase activity of mycobacterial plasma membrane was assessed. We performed bioinformatic analyses which indicated that the pma1 gene product is the M. smegmatis ortholog of the M. tuberculosis cation transporter CtpF. In addition, a possible Na+, K+ and/or Ca2+ pumping mediated by Pma1 was also predicted. Enzymatic analyses indicated that the basal ATPase activity of plasma membrane vesicles from M. smegmatis cells cultured under hypoxia and over-expressing DosR, decreased 30 and 40 % respectively in comparison to oxygenated cells. In contrast, the specific Na+/K+ and Ca2+ ATPase activities of the plasma membrane increased 2.8- and 3.5-fold, respectively, under hypoxia, similar to that observed for cells over-expressing the DosR regulator. In agreement, RT-qPCR experiments demonstrated that the transcription level of the pma1 gene increased under hypoxia at levels similar to that of M. smegmatis cells over-expressing the M. tuberculosis DosR regulator. The entire findings suggest that hypoxia stimulates Na+/K+ and Ca2+ ATPase activities in the mycobacterial plasma membrane, and this is possibly mediated by the dormancy regulator DosR.



Similar content being viewed by others
References
Agranoff DD, Krishna S (1998) Metal ion homeostasis and intracellular parasitism. Mol Microbiol 28(3):403–412
Andersen JP, Vilsen B (1995) Structure-function relationships of cation translocation by Ca2+- and Na+, K+-ATPases studied by site-directed mutagenesis. FEBS Lett 359(2–3):101–106
Bacon J, James BW, Wernisch L, Williams A, Morley KA, Hatch GJ, Mangan JA, Hinds J, Stoker NG, Butcher PD, Marsh PD (2004) The influence of reduced oxygen availability on pathogenicity and gene expression in Mycobacterium tuberculosis. Tuberculosis (Edinb) 84(3–4):205–217
Bartek IL, Rutherford R, Gruppo V, Morton RA, Morris RP, Klein MR, Visconti KC, Ryan GJ, Schoolnik GK, Lenaerts A, Voskuil MI (2009) The DosR regulon of M. tuberculosis and antibacterial tolerance. Tuberculosis (Edinb) 89(4):310–316
Basu J, Chattopadhyay R, Kundu M, Chakrabarti P (1992) Purification and partial characterization of a penicillin-binding protein from Mycobacterium smegmatis. J Bacteriol 174(14):4829–4832
Berney M, Cook GM (2010) Unique flexibility in energy metabolism allows mycobacteria to combat starvation and hypoxia. PLoS ONE 5(1):e8614
Botella H, Peyron P, Levillain F, Poincloux R, Poquet Y, Brandli I, Wang C, Tailleux L, Tilleul S, Charriere GM, Waddell SJ, Foti M, Lugo-Villarino G, Gao Q, Maridonneau-Parini I, Butcher PD, Castagnoli PR, Gicquel B, de Chastellier C, Neyrolles O (2011) Mycobacterial P(1)-type ATPases mediate resistance to zinc poisoning in human macrophages. Cell Host Microbe 10(3):248–259
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Bua A, Rosu V, Molicotti P, Das Gupta SK, Ahmed N, Zanetti S, Sechi LA (2009) Phages specific for mycobacterial lipoarabinomannan help serodiagnosis of tuberculosis. New Microbiol 32(3):293–296
Bublitz M, Poulsen H, Morth JP, Nissen P (2010) In and out of the cation pumps: P-type ATPase structure revisited. Curr Opin Struct Biol 20(4):431–439
Cook GM, Berney M, Gebhard S, Heinemann M, Cox RA, Danilchanka O, Niederweis M (2009) Physiology of mycobacteria. Adv Microb Physiol 55(81–182):189–318
Fiske SHaS Y (1925) The colorimetric determination of phosphorus. J Biol Chem 66(2):375–400
Gerasimova A, Kazakov AE, Arkin AP, Dubchak I, Gelfand MS (2011) Comparative genomics of the dormancy regulons in mycobacteria. J Bacteriol 193(14):3446–3452
Jha SS, Danelishvili L, Wagner D, Maser J, Li YJ, Moric I, Vogt S, Yamazaki Y, Lai B, Bermudez LE (2010) Virulence-related Mycobacterium avium subsp hominissuis MAV_2928 gene is associated with vacuole remodeling in macrophages. BMC Microbiol 10:100
Kim MJ, Park KJ, Ko IJ, Kim YM, Oh JI (2010) Different roles of DosS and DosT in the hypoxic adaptation of Mycobacteria. J Bacteriol 192(19):4868–4875
Kimura M, Yamaguchi Y, Takada S, Tanabe K (1993) Cloning of a Ca2+-ATPase gene of Plasmodium falciparum and comparison with vertebrate Ca2+-ATPases. J Cell Sci 104(Pt 4):1129–1136
Kobayashi H, Anraku Y (1972) Membrane-bound adenosine triphosphatase of Escherichia coli. I. Partial purification and properties. J Biochem 71(3):387–399
Kremer L, Guerardel Y, Gurcha SS, Locht C, Besra GS (2002) Temperature-induced changes in the cell-wall components of Mycobacterium thermoresistibile. Microbiology 148(Pt 10):3145–3154
Kruh NA, Troudt J, Izzo A, Prenni J, Dobos KM (2010) Portrait of a pathogen: the Mycobacterium tuberculosis proteome in vivo. PLoS ONE 5(11):e13938
Kumar M, Khan FG, Sharma S, Kumar R, Faujdar J, Sharma R, Chauhan DS, Singh R, Magotra SK, Khan IA (2011) Identification of Mycobacterium tuberculosis genes preferentially expressed during human infection. Microb Pathog 50(1):31–38
Leistikow RL, Morton RA, Bartek IL, Frimpong I, Wagner K, Voskuil MI (2010) The Mycobacterium tuberculosis DosR regulon assists in metabolic homeostasis and enables rapid recovery from nonrespiring dormancy. J Bacteriol 192(6):1662–1670
Morth JP, Pedersen BP, Toustrup-Jensen MS, Sorensen TL, Petersen J, Andersen JP, Vilsen B, Nissen P (2007) Crystal structure of the sodium-potassium pump. Nature 450(7172):1043–1049
Murphy DJ, Brown JR (2007) Identification of gene targets against dormant phase Mycobacterium tuberculosis infections. BMC Infect Dis 7:84
Novoa-Aponte L, Leon-Torres A, Patino-Ruiz M, Cuesta-Bernal J, Salazar LM, Landsman D, Marino-Ramirez L, Soto CY (2012) In silico Identification and characterization of the ion transport specificity for P-type ATPases in the Mycobacterium tuberculosis complex. BMC Struct Biol 12(1):25
Ogawa H, Toyoshima C (2002) Homology modeling of the cation binding sites of Na+ K+-ATPase. Proc Natl Acad Sci USA 99(25):15977–15982
Palmgren MG, Nissen P (2011) P-type ATPases. Ann Rev Biophys 40:243–266
Park HD, Guinn KM, Harrell MI, Liao R, Voskuil MI, Tompa M, Schoolnik GK, Sherman DR (2003) Rv3133c/dosR is a transcription factor that mediates the hypoxic response of Mycobacterium tuberculosis. Mol Microbiol 48(3):833–843
Raeymaekers L, Wuytack E, Willems I, Michiels CW, Wuytack F (2002) Expression of a P-type Ca2+-transport ATPase in Bacillus subtilis during sporulation. Cell Calcium 32(2):93
Raimunda D, Long JE, Sassetti CM, Arguello JM (2012) Role in metal homeostasis of CtpD, a Co2+ transporting P(1B4)-ATPase of Mycobacterium smegmatis. Mol Microbiol 84(6):1139–1149
Roberts DM, Liao RP, Wisedchaisri G, Hol WG, Sherman DR (2004) Two sensor kinases contribute to the hypoxic response of Mycobacterium tuberculosis. J Biol Chem 279(22):23082–23087
Santos P, Gordillo A, Osses L, Salazar LM, Soto CY (2012) Effect of antimicrobial peptides on ATPase activity and proton pumping in plasma membrane vesicles obtained from mycobacteria. Peptides 36(1):121–128
Schnappinger D, Ehrt S, Voskuil MI, Liu Y, Mangan JA, Monahan IM, Dolganov G, Efron B, Butcher PD, Nathan C, Schoolnik GK (2003) Transcriptional Adaptation of Mycobacterium tuberculosis within Macrophages: insights into the Phagosomal Environment. J Exp Med 198(5):693–704
Somerville W, Thibert L, Schwartzman K, Behr MA (2005) Extraction of Mycobacterium tuberculosis DNA: a question of containment. J Clin Microbiol 43(6):2996–2997
Stover CK, de la Cruz VF, Fuerst TR, Burlein JE, Benson LA, Bennett LT, Bansal GP, Young JF, Lee MH, Hatfull GF et al (1991) New use of BCG for recombinant vaccines. Nature 351(6326):456–460
Thever MD, Saier MH Jr (2009) Bioinformatic characterization of P-type ATPases encoded within the fully sequenced genomes of 26 eukaryotes. J Membr Biol 229(3):115–130
Trias J, Benz R (1994) Permeability of the cell wall of Mycobacterium smegmatis. Mol Microbiol 14(2):283–290
Wagner D, Maser J, Lai B, Cai Z, Barry CE 3rd, Honer Zu, Bentrup K, Russell DG, Bermudez LE (2005) Elemental analysis of Mycobacterium avium-, Mycobacterium tuberculosis-, and Mycobacterium smegmatis-containing phagosomes indicates pathogen-induced microenvironments within the host cell’s endosomal system. J Immunol 174(3):1491–1500
Wayne LG, Hayes LG (1996) An in vitro model for sequential study of shiftdown of Mycobacterium tuberculosis through two stages of nonreplicating persistence. Infect Immun 64(6):2062–2069
WHO (2012) Global tuberculosis report 2012. World Health Organization, Switzerland
Wiangnon K, Raksajit W, Incharoensakdi A (2007) Presence of a Na+-stimulated P-type ATPase in the plasma membrane of the alkaliphilic halotolerant cyanobacterium Aphanothece halophytica. FEMS Microbiol Lett 270(1):139–145
Yatime L, Buch-Pedersen MJ, Musgaard M, Morth JP, Lund Winther AM, Pedersen BP, Olesen C, Andersen JP, Vilsen B, Schiott B, Palmgren MG, Moller JV, Nissen P, Fedosova N (2009) P-type ATPases as drug targets: tools for medicine and science. Biochim Biophys Acta 1787(4):207–220
Zhang L, Zhong Q, Bao L, Zhang Y, Gao L, Huang B, Zhang HD (2009) Rv0901 from Mycobacterium tuberculosis, a possible novel virulent gene proved through the recombinant Mycobacterium smegmatis. Jpn J Infect Dis 62(1):26–31
Acknowledgments
This work was supported by the “División de Investigación Bogotá, DIB”, Vicerrectoría de Investigación, Universidad Nacional de Colombia, grants 15835, 14337, 14837 and 16060.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Online Resource 1
HMM search results for the M. smegmatis mc2155 P-type ATPases. Probabilistic profiles based on hidden Markov models were constructed to identify and classify Pma1 and CtpF according to the possible ion that is pumped across the plasma membrane. The plot was constructed using the scores that were obtained using the Hmmsearch tool. Those scores show the similarity between the identified sequences in the M. smegmatis mc2155 proteome and the grouped sequences that were used for each HMM construction. The gene products of M. smegmatis pma1 and M. tuberculosis ctpF are possible alkali or alkaline/earth metal cation transporters. Both proteins, CtpF and Pma1, had the highest amino acid sequence homology to vertebrate sarcoplasmic/endoplasmic reticulum Ca2+ATPases (SERCA), and comparable homology to plasma membrane Ca2+ ATPases and Na+/K+ ATPases (DOCX 304 kb)
Online Resource 2
Hydrophobicity analyses of the M. smegmatis pma1 gene product. The amino acid sequences of M. tuberculosis CtpF and M. smegmatis Pma1 were analysed using the TMHMM 2.0 tool. Both proteins exhibited 10 transmembrane segments that were arranged into a type II topology (DOCX 55 kb)
Rights and permissions
About this article
Cite this article
Pulido, P.A., Novoa-Aponte, L., Villamil, N. et al. The DosR Dormancy Regulator of Mycobacterium tuberculosis Stimulates the Na+/K+ and Ca2+ ATPase Activities in Plasma Membrane Vesicles of Mycobacteria. Curr Microbiol 69, 604–610 (2014). https://doi.org/10.1007/s00284-014-0632-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-014-0632-6