Abstract
In the galectin family, a group of lectins is united by their evolutionarily conserved carbohydrate recognition domains. These polypeptides play a role in various cellular processes and are implicated in disease mechanisms such as cancer, fibrosis, infection, and inflammation. Following synthesis in the cytosol, manifold interactions of galectins have been described both extracellularly and intracellularly. Extracellular galectins frequently engage with glycoproteins or glycolipids in a carbohydrate-dependent manner. Intracellularly, galectins bind to non-glycosylated proteins situated in distinct cellular compartments, each with multiple cellular functions. This diversity complicates attempts to form a comprehensive understanding of the role of galectin molecules within the cell. This review enumerates intracellular galectin interaction partners and outlines their involvement in cellular processes. The intricate connections between galectin functions and pathomechanisms are illustrated through discussions of intracellular galectin assemblies in immune and cancer cells. This underscores the imperative need to fully comprehend the interplay of galectins with the cellular machinery and to devise therapeutic strategies aimed at counteracting the establishment of galectin-based disease mechanisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The glycome contains important biological information and in this way has a major impact on the cellular function and behaviour [1]. Cell tools for glycome manipulation employ glycosyl transferases, glycosidases and lectins. A β-galactoside binding lectin family are the galectins [2]. They have been found in multicellular organisms and can be detected intra- as well as extracellularly. Numerous functions attributed to galectins revolve around extracellular carbohydrate recognition, targeting either glycosylated membrane components or soluble ligands. However, it is noteworthy that galectins are synthesized in the cytosol, a cellular compartment where they can engage in specific interactions with various binding partners. In this intracellular environment, galectins establish connections with cellular entities, showcasing their versatility in influencing processes beyond the realm of extracellular carbohydrate recognition.
The galectin family
The first galectin was discovered 1975 in the electric eel (Electrophorus electricus) and was named electrolectin [3]. The term galectin was introduced in 1994 [2]. So far 19 galectin subtypes have been identified. These lectins share distinctive primary amino acid sequences and are characterized by containing one or two carbohydrate recognition domains (CRD). Based on the CRD-number and allocation, galectins were classified into three groups. Prototype galectins contain only one CRD domain and can dimerize by non-covalent binding (galectin-1, 2, 5, 7, 10, 11, 13, 14 and 16) (Fig. 1). Tandem repeat-type galectins are equipped with two distinct CRDs with individual sugar-binding capacities, which are linked by a stretch of 5 to 50 amino acids (galectin-4, 6, 8, 9 and 12). The sole member of the chimera-type galectins is galectin-3. This individual variant contains one CRD that is linked to a non-lectin proline-rich N-terminal domain. Galectins found in the human genome were classified by the HUGO Gene Nomenclature Committee into galectin-1, -2, -3, -4, -7, -7B, -8, -9, -9B, 9 C, -10, -12, -13, -14, and − 16 (Gene group: Galectins (LGALS)).
Domain structure of galectin subtypes. Prototype galectins contain one CRD, galectin-1, -2, -5, -7, -10, -11, -13, -14 and − 16. They are often characterized by a dimeric quaternary structure. Tandem repeat-type galectin-4, -6, -8, -9 and 12 are characterized by two CRDs. The only chimera-type galectin with a dynamic proline/tyrosine/glycine-repeat N-terminal domain and one C-terminal CRD is galectin-3. Type-C and Type-N-self-association by the C- or N-terminal domain into oligomeric assemblies have been described
The CRD contains all amino acid residues associated with the carbohydrate binding and is assembled from two extended antiparallel β-sheets folded into a β-sandwich structure [4]. Phylogenetic CRD-analysis based on genomic and mRNA data revealed that galectins with two CRDs emerged from an original bi-CRD galectin, which arose by tandem duplication of the gene encoding the ancestral mono-CRD galectin [5]. The β-sandwich CRD structure is widespread and many viral glycan-binding proteins contain a similar structural architecture so that it is likely that these viral proteins originate from host lectins [6]. Galectins specifically interact with N-acetyllactosamine (LN, Galβ1,3GlcNAc or Galβ1,4GlcNAc), a component present in various N- or O-linked glycans found on proteins throughout the cell. The concentrations of lectins required for half-maximal saturation of LN-binding, also known as the equilibrium dissociation constant (Kd), typically range from ten to several hundred µM. For example, galectin-1 exhibits a Kd of 21.5 µM [7], galectin-2 has a Kd of 654 µM [8], Kds for galectin-3 range from 27.9 to 93 µM [8, 9], full length galectin-4 has a Kd of 9.3 µM [8] and galectin-7 has a Kd within the range of 270–410 µM [9]. Their affinity for binding to oligosaccharides is higher compared to monosaccharides, and this affinity increases with the number of glycans [10]. In general, galectin binding affinities for most oligosaccharidic structures is relatively low. This can be compensated by galectin-oligomerization to form multivalent interactions with target molecules [11]. Some galectins have a preferential tissue specific localization like galectin-7 in the skin, galectin-10 in eosinophils/regulatory T cells, galectin-12 in adipose tissue [12] and galectin-13, 14 as well as 16 in the placenta [13, 14]. Other galectins like galectin-1 and galectin-3 show a more ubiquitous distribution.
Here, we primarily focus on the interactions of galectins with distinct polypeptides in intracellular compartments under normal and pathological conditions.
Galectin interactions in the nucleus
Some galectins possess classical nuclear localization sequences, resulting in their translocation from the cytosol into the nucleus (see Fig. 2). Examples include murine galectin-3 with the sequence (253)ITLT(256) [15] and human galectin-3 with (223)HRVKKL(228) [16]. The latter interacts with importin-α to facilitate passage through nuclear pores (refer to Table 1). Murine galectin-3’s N-terminus also demonstrates active nuclear transport capabilities, featuring a leucine-rich nuclear export signal (NES) at positions 241–249 [17]. Significantly, the phosphorylation of galectin-3 at Ser-6 by casein kinase 1 (CK1) appears to be involved in its nuclear export and anti-apoptotic activity, as illustrated in Fig. 2 [18, 19]. Phosphorylated galectin-3 is translocated from the nucleus to the cytosol, providing protection to the cells against drug-induced apoptosis. Consequently, the phosphorylation of galectin-3 at Serine-6 functions as a molecular switch, regulating its cellular translocation from the nucleus to the cytosol. Additionally, the pool of nuclear galectin-3 is augmented by a proline to histidine substitution at position 64, observed in breast and gastric cancers, thereby promoting cancer progression [20, 21].
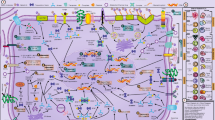
Intracellular binding partners of galectins in distinct cellular processes. Different cellular localizations and interaction partners of galectins are exemplified on a scheme depicting cellular organelles. Subtypes of prototype and tandem repeat galectins are indicated. E, endosome; LT, lactotransferrin; MVE, multivesicular endosome; SE, sorting endosome; V, transport vesicle
Within the nucleus, galectin-3 is localized in nuclear speckles, as evidenced by its co-localization with the Ser-, Arg-rich splicing factor SC35 [22, 23]. These nuclear dots, in terms of both number and size, resemble two subnuclear domains known as Cajal bodies and gems (coiled Cajal bodies). Galectin-3, along with galectin-1, interacts with the gem marker protein Gemin4 (Fig. 2), as demonstrated through GST pulldown or immunoprecipitation experiments [24, 25]. Both of these galectins play roles in nuclear splicing of pre-mRNAs and gene expression [26, 27]. They have been shown to function redundantly as splicing factors for mRNA processing and nuclear export [28]. In human umbilical vein endothelial cells (HUVECs), the knockdown of galectin-1 has a notable impact on the expression of angiogenesis-related genes [29]. Through interactome and transcriptome analysis in this study, it was revealed that galectin-1 serves as a potent regulator of alternative splicing patterns for angiogenesis-associated genes, particularly those involved in focal adhesion and the angiogenesis-associated vascular endothelial growth factor signaling. The lectin may influence the transcriptome profiles of HUVECs by binding to RNA transcripts. Notably, using a galectin-1 mutant deficient in carbohydrate binding, Voss et al. demonstrated that carbohydrate binding is not implicated in the interaction of galectin-1 with spliceosomal components [30].
The sequence of events in galectin-interactions in spliceosome assembly has been studied in detail for galectin-3. When splicing is initiated, heterogeneous nuclear ribonucleoproteins (hnRNPs) assemble on the pre-mRNA to form the H complex (Fig. 2). One component of this complex is hnRNPA2B1, which interacts with galectin-3 [23]. If the N-terminal galectin-3-domain is added pre-mRNA splicing is arrested at a position corresponding to the H-complex, thus suggesting a functional role of galectin-3 in the first steps of splicing initiation [24]. Co-immunoprecipitation of galectin-3 from nuclear extracts also revealed components of the pre-spliceosome complexes E and A [23]. Evidence for interaction of galectin-3 with complex E came from earlier studies. This lectin fractionated in glycerol gradient separation studies from nuclear extracts in pre-mRNA particles together with the small nuclear ribonucleoprotein (snRNP) U1 thus forming a functional complex E [25, 31]. Galectin-3 itself is not a classical RNA-binding protein because it does not contain an RNA recognition motif [32]. Nevertheless, association of galectin-3 with snRNP U1 depends on the integrity of the snRNA of U1 and is affected in the presence of lactose. Together, these data strongly suggest that galectin-3 is involved in the initiation of spliceosome assembly and the splicing cascade.
In addition, mature mRNA can indirectly interact with galectin-3 in the perinuclear region. This has been demonstrated in pancreatic cancer cells, where the lectin binds to the hnRNP-L [32] (Fig. 2). It stabilizes the mRNA of the membrane-associated mucin MUC4, which activates genes involved in cell proliferation [33]. Expression of MUC2 is triggered by galectin-3 in intestinal cells by a different mechanism. Here, galectin-3 associates with c-Jun and Fra-1 in the nucleus to form an activating transcription factor complex at the AP-1 binding site on the MUC2 promoter [34]. Another example of galectin-3 as a transcription effector is provided by interaction with the HSF-1 transcription factor to promote expression of the cell adhesion molecule neogenin-1, cell survival in gastric cancer and cell motility [35](Fig. 2). Finally, involvement of galectin-3 in the cellular transcription machinery is demonstrated by its interaction with the transcription factor Sp1 in hematopoietic stem cells to upregulate p21 expression and control cell cycle progression [36].
Hox family proteins are transcription factors participating in embryonal development by gene expression regulation. Pull-down experiments revealed that placental galectin-13 binds to HOXA1, one of the earliest Hox genes to be expressed during embryonic development [37, 38]. HOXA1 can interact with the monomeric form of galectin-13 in the nucleus (Fig. 2). However, binding to dimeric galectin-13, which can be stabilized by disulphide bonds [39], is more efficient. It will be interesting to see future research on the consequences of this interaction on the cellular transcriptome especially in embryonic development. There is still another published nuclear galectin interaction that requires further characterization. With a sequence identity of about 60%, the two homologues prototype galectins galectin-14 and galectin-16 interact with the NF-κB family transcription factor c-Rel [40,41,42](Fig. 2). Consequences of this interaction may affect signal transduction, but the precise mechanistic details require further investigation.
Cytosolic galectin interactions and non-classical galectin secretion
The functions of galectins are diverse and involve intricate interactions within cellular processes. In cellular signalling, galectin-1 is crucial for the nanoclustering of the small GTPase H-Ras [43, 44], while galectin-3 plays a similar role for K-Ras [45,46,47](Fig. 2). These nanoclusters, although short-lived, assemble on the cytoplasmic membrane leaflet, influencing signaling cascades. Galectin-1 specifically binds to and stabilizes dimers of the Raf effector, positively regulating GTP-H-Ras nanoscale signaling hubs [48]. A recent atomistic structural model of the Ras-Raf signalosome, including galectin-3 and 14-3-3σ, provides structural insight into these interactions [49]. In one scenario, galectin-3, capable of associating with the lipid bilayer [50], caps the farnesyl group of K-Ras in a hydrophobic binding pocket, facilitating the membrane localization of K-Ras assemblies. Notably, there is no evidence suggesting the involvement of sugar moieties in these cytosolic galectin functions. Conversely, galectin-3 associates with the nuclear mitotic apparatus protein (NuMA) in a sugar-dependent fashion [51] (Fig. 2). NuMA is a key mitotic regulator crucial for spindle pole establishment and cohesion. A mutant NuMA form blocked in O-GlcNAc-glycosylation leads to aberrant spindle pole formation. This mutant does not co-immunoprecipitate galectin-3, which suggests that the galectin-3-NuMA interaction depends on O-GlcNAc glycosylation. The exact mechanism remains unclear as O-GlcNAc does not bind to galectin-3 by itself. The cytosolic interaction of galectin-3 with basal bodies and centrosomes is significant, as depletion or knockdown of galectin-3 results in microtubule disorganization, perturbation of epithelial morphogenesis, and disorganization of primary cilia [52] or motile respiratory kinocilia [53].
It is interesting to note that in Hela cells galectin-3 can by itself be O-GlcNAcylated in the cytoplasm by O-GlcNAc transferase (OGT) [54]. This glycan modification, rarely found on secreted galectin-3 polypeptides, suggests that O-GlcNAcylation might trigger export of the lectin from the cytoplasm into the extracellular milieu. Additionally, tyrosine-phosphorylation by members of the Src kinase family affects the secretion of galectin-4 [55].
In general, secretion of galectins does not involve the classical transport pathway starting at the rough endoplasmic reticulum followed by vesicular transport to the Golgi apparatus, the trans Golgi network and endosomal compartments up to the plasma membrane. Instead, they are secreted by so-called non-classical mechanisms. One non-classical secretion scenario describes the sorting of cargo by members of the endosomal complex required for transport (ESCRT) into intraluminal vesicles (ILVs) at the membrane of multivesicular endosomes (MVEs) [56](Fig. 2). Galectin-3 interacts in the cytosol with Tsg101, a component of the ESCRT-I complex [57]. This direct interaction, facilitated by a highly conserved tetrapeptide P(S/T)AP motif (late domain motif) in the amino terminus of galectin-3, ensures recruitment into ILVs. The fusion of multivesicular endosomes (MVE) with the plasma membrane leads to the release of these vesicles as extracellular vesicles (EVs), functioning as communication platforms between cells or even organs within a living organism. The membrane protein E-cadherin is integrated into EV membranes through a comparable mechanism [58].
The primary secretion mechanism for galectin-3 from the apical membrane of polarized kidney epithelial cells is via EV-based secretion. Evidence for a functional role of cell-to-cell transfer of galectin-3-containing EVs comes from a study on the communication between B-cell precursor acute lymphoblastic leukemia cells and stromal cells [59]. Fei et al. discovered that galectin-3 released from stromal cells is internalized by target cells and transported into the cell nucleus. This process induces galectin-3 expression and provides protection to the target cells against drug treatment.
While a late domain motif has not been identified in other galectin-family members, the presence of galectin-1 [60], galectin-4 [61], galectin-5 [62] galectin-9 [61] and galectin-13 [63] in extracellular vesicle pools suggests recruitment by yet unidentified mechanisms. Interaction of galectin-13 with cytosolic annexin II at the plasma membrane may play functional roles in the non-classical secretion of this lectin from placenta and fetal hepatic cells [39](Fig. 2). Alternative pathways for non-classical secretion of galectins are also under discussion. The interaction capacity of galectin-3 with membrane lipids suggests the possibility of spontaneous penetration of lipid bilayers [50]. The whole matter of non-classical secretion of galectins has not been settled yet and further studies are required to fully understand all processes involved. Notably, galectin-10 is released by eosinophil cells through a process of active cell death referred to as ETosis, elevating serum levels of galectin-10 [64]. This lytic degranulation of eosinophile cells elevates serum levels of galectin-10.
Galectin binding partners in endosomal, lysosomal or vesicular lumina
Galectins can be secreted and endocytosed or recruited into MVEs to reside within endosomes, lysosomes or transport vesicles, where they function as mechanistic components of vesicular trafficking. Internalized galectin-3, -4 and − 9 are involved in the apical endocytic-recycling of newly synthesized glycoproteins [65,66,67,68,69](Fig. 2). While being transported from the trans Golgi network (TGN) to the plasma membrane, galectin-3 binds to glycans that are exposed on the neurotrophin receptor. This binding leads to the clustering of the neurotrophin receptor, a non-raft-associated glycoprotein, into high molecular weight clusters. These clusters are then recruited into apical transport vesicles [70]. Galectin-3 is involved in apical targeting of the carcinoembryonic antigen-related cell adhesion molecule 1 (CAECAM1), dipeptidylpeptidase IV (DPPIV), lactase-phlorizin hydrolase (LPH), the neurotrophin receptor (p75), sodium-proton exchanger 2 (NHE2) and integrin-β1 [71,72,73]. A distinct set of lipid raft-associated apical cargo molecules is sorted by galectin-4 into apical transport carriers [66, 69]. In addition to binding to glycoproteins, the two carbohydrate recognition domains (CRDs) of galectin-4 interact specifically and with high affinity with a defined pool of glycosphingolipids. Specifically, this interaction involves sulfatides with long fatty acid chains, hydroxylated in position-2. The binding of galectin-4 to raft constituents results in cross-linking, forming raft clusters. These clusters are postulated to contribute to the formation of membrane microdomains that subsequently bud off to generate apical transport carriers. Once at the apical membrane, galectins can undergo re-internalization. It’s worth noting that the efficiency of galectin-3 internalization is pH-dependent and can be competitively inhibited by lactose [74]. Recent evidence indicates that the sialomucin podocalyxin establishes a ligand-receptor pair with galectin-3, initiating the co-internalization of both molecules from the apical membrane [75](Fig. 2). The endocytic process involves galectin-3-mediated cargo clustering and membrane bending, playing a functional role that is dependent on glycosphingolipids, as demonstrated in prior studies [76]. In mouse enterocytes, a glycosphingolipid-dependent internalization of lactoferrin and galectin-3 from the apical membrane has been previously described [77]. It also seems plausible that vesicular recycling of galectin-3 between the plasma membrane and endosomal organelles is maintained by alterations in the interaction partners. This view is supported by pH-dependent fine-tuning of galectin-3 cluster formation [78]. Moreover, the interaction between galectin-3 and integrin-β1, as well as galectin-4 with the transferrin receptor, facilitates basolateral to apical epithelial transcytosis [73, 79](Fig. 2). The elimination of these interactions results in the lysosomal targeting of the associated cargo molecules. This observation suggests that galectin-mediated apical sorting is not limited to newly synthesized cargo molecules but extends to previously existing ones as well.
Galectin-9 is enriched in the lumen of lysosomes from enterocytes and predominantly binds to the lysosome-associated membrane protein 2 (Lamp2) [80](Fig. 2). This lectin interaction, largely with the N-glycan chain attached to Asn-175 of Lamp2, stabilizes lysosomes, maintains lysosomal acidity, and facilitates lysosome-mediated autophagy to prevent ER stress and associated cell apoptosis in intestinal Paneth cells and acinar cells of the pancreas. In dendritic cells, mast cells and macrophages galectin-9 is involved in cytokine secretion [81,82,83]. Intracellular galectin-9 interaction with the soluble NSF attachment receptor vesicle associated membrane protein 3 (VAMP3) regulates cytokine secretion in dendritic cells [83](Fig. 2). Interestingly, galectin-9 binds to the C-terminus of cytosolic sorting nexin-3 (SNX3) in macrophages to regulate compaction of intact Borrelia-containing phagosomes, thus driving their maturation and Borrelia degradation [84]. However, the exact role of galectin-9 in the curvature-sensitive process of Borrelia phagosome compaction is not known yet.
The Charcot-Leyden crystal component galectin-10 interacts with glycosylated human eosinophil granule cationic RNAses, namely, eosinophil-derived neurotoxin (RNS2) and eosinophil cationic protein (RNS3), in a glycan-independent fashion [85]. Lectin-interaction does not inhibit the endoRNAses, but may play a role in their vesicular transport from granulogenesis until secretion in mature eosinophile cells.
Galectin binding involved in lysosomal damage
Recent evidence points on the involvement of galectins in lysosomal homeostasis. Lysosomes can be damaged by exposure to biologically active silica crystals, proteopathic fibrils or microbial invasion. Once damaged lysosomes are beyond repair, they are removed by autophagy (for review see [86]). In this process galectins in conjunction with ubiquitination serve as “eat me” signals to control orchestration of several autophagy regulators. They recognize membrane damage by binding to lumenal β-galactosides once glycoconjugates on the exofacial leaflet are exposed to the cytosol and bind to and recruit autophagic receptors. Initial publications of this phenomenon describe binding of galectin-3 to host glycans exposed on lysed vacuoles following Shigella, Listeria and Salmonella infection [87]. Thereafter, it was shown that galectin-3, -8 and − 9 sense the exposure of host glycans on membranes ruptured by Listeria, Salmonella and Shigella infection [88]. Mechanistic insight was provided by the finding that binding of the autophagic receptor NDP52 to the C-terminal CRD of galectin-8 and ubiquitin targets damaged Salmonella-containing vacuoles and activates antibacterial autophagy (Fig. 2). Moreover, in response to lysosomal damage galectin-8 dynamically associates with and inactivates the Ser/Thr protein kinase mTOR, which acts as a negative regulator by phosphorylating inhibitory sites on regulators of autophagy [89]. In conjunction with proximity biotinylation proteomics data these observations indicate that galectin-8 is a key regulatory node for lysophagy [90]. Galectin-9, which is also important for an optimal autophagic response, activates the AMP-activated protein kinase (AMPK) by displacement of the deubiquitinase USP9X from AMPK-TAK1-complexes to promote ubiquitination of TAK1 [89, 91]. The sequence of events in the action of galectin-3 on damaged lysosomes starts with targeting of the lectin to damage-exposed glycans. Here, interaction of the lectin with glycans exposed on the transferrin receptor (CD71) is functionally important for Gal3 recruitment to damaged lysosomes [92](Fig. 2). Galectin-3 then interacts with the ESCRT component ALG-2-interacting protein X (ALIX) [92,93,94] to facilitate ESCRT-III complex formation and mediate lysosomal repair [95, 96]. The intriguing aspect is that the interaction between galectin-3 and ALIX intensifies as lysosomal damage progresses. Simultaneously, the interaction with the ESCRT-I component Tsg101 decreases. This observation suggests a scenario in which ALIX selectively interacts with galectin-3 to facilitate lysosomal repair, while the association with Tsg101 aids in recruiting the lectin into multivesicular endosomes (MVEs) for extracellular vesicle (EV) secretion. At a later step, when damaged lysosomes have to be removed by autophagy, galectin-3 switches from ALIX to the tripartite motif protein TRIM16 [97]. Here, the CRD-domain of galectin-3 interacts with TRIM16 to recognise endomembrane damage and associate with the key autophagy regulators ATG16L1, ULK1 and Beclin 1. They govern cargo degradation in a highly precise process [98]. Very recently, interaction of galectin-3 with the lysophagy receptor coiled-coil domain-containing 50 (CCDC50) has been demonstrated to control lysosomal integrity and renewal [99]. This interaction may not be direct. In melanoma CCDC50 is upregulated and the galectin-3/CCDC50-mediated lysophagy mechanism supports tumour growth and, potentially, also works in other tumours. Thus, especially within the last decade many aspects in the complex network of galectin interaction partners in lysosomal damage and autophagy have been uncovered to yield a cohesive view of galectin activities in this field.
Intracellular galectin interactions in immune cells
Extracellular galectin-1 and galectin-3 are both inducing T cell apoptosis by interaction with glycoprotein receptors CD45 and CD71 on the cell surface, which weakens the T cell mediated immune response [100]. It is thus surprising that intracellular galectin-3 has the opposite effect on T cell apoptosis. In Jurkat cells the carbohydrate binding domain of galectin-3 intracellularly interacts with the apoptosis inhibitor Bcl-2 to suppress apoptosis and promote cell growth [101]. Furthermore, Mohammadpour et al. demonstrated that the expression of galectin-3 provides partial protection to T cells against apoptosis induced by IFN-γ [102]. It remains unclear whether this protection is contingent upon extracellular or intracellular interactions of galectin-3. Nevertheless, antiapoptotic galectin-3 function is consistent with its known anti-apoptotic role in other cell types, including cancer cells [103]. The study of Mohammadpour et al. also shows that galectin-3 acts as a negative regulator of T cell function and proliferation [102]. The conflicting impacts, with its antiapoptotic properties on one side and the dampening of the immune response on the other, highlight the nuanced role of galectin-3 in T cells. A documented attenuation of the immune response by intracellular galectin-3 has specifically been observed in activated T cells. Here, galectin-3 interaction with the ESCRT component ALIX is focussed to the stable contact region between T cells and antigen-presenting cells (APCs) called the immunological synapse (IS) [93]. This cytoplasmic interaction may promote T cell receptor downregulation and T cell inactivation by attenuation of ALIX-mediated trafficking events. In addition, Kaur et al. describe an increase in intracellular galectin-3 upon activation of CD8+ T cells following herpesvirus infection in comparison to the secreted galectin-pool [104]. In activated CD8+ T cells, cytoplasmic galectin-3 is mobilized to the immunological synapse (IS), where it diminishes both their proliferation and cytokine production, thereby impeding T cell functions. In contrast, galectin-9 exerts a positive influence on the immune response. It is recruited intracellularly to the IS upon T cell activation and promotes T cell receptor signalling, which affects T cell differentiation and B cell responses [105]. The precise mechanism of this galectin-9 mediated function, however, has not been clarified yet. Dendritic cells express galectin-1 which intracellularly interacts with the cytoplasmic adaptor protein Bam32 once the cell has reached the mature state [106](Fig. 2). This interaction might modulate the stimulatory capacity of mature dendritic cells to activate CD8+ T cells. Moreover, galectin-9 is also expressed in dendritic cells and has been shown to organize the cortical actin cytoskeleton to optimize their phagocytic capacity [107]. Knockdown experiments suggest that the underlying mechanism is based on a galectin-9 mediated promotion of Rac1-GTP activation, which then reorganizes the actin cytoskeleton and affects membrane rigidity.
Intracellular interactions of galectin-3 also suppress immune responses in neutrophil and dendritic cells. In neutrophil cells galectin-3 inhibits the neutrophil ROS response to phorbol myristate acetate or zymosan stimulation and regulates the life span of neutrophils during virulent Toxoplasma infection [108]. Importantly, mechanistic insight into the role of galectin-3 in attenuation of neutrophil cells came from a subsequent study on phagocytosis of Candida [109]. Wu et al. found that galectin-3 physically interacts with the tyrosine kinase Syk (Fig. 2), which suppresses complement receptor 3 (CR3) downstream Syk-mediated ROS production and anti-Candida function of neutrophil cells. Another study with dendritic cells did not observe galectin-3 mediated effects on Syk-induced signalling after stimulation of dectin-1 or TLR4, two different classes of pattern recognition receptors that identify molecular components expressed on microbial pathogens [110]. Instead, they demonstrate that galectin-3 affects cytokine expression of dendritic cells to suppress T cell responses. These observations are in line with data from Leishmania infected knockout mice showing that galectin-3 deficiency leads to increased activation of the Notch signalling pathway, and enhanced production of both pro- and anti-inflammatory cytokines [111]. Some of the observed effects in these studies can be explained by extracellular interaction of galectin-3 with glycan moieties exposed on the extracellular receptor domains. Nevertheless, the finding that neither the presence of lactose nor the addition of recombinant galectin-3 to dendritic cell cultures affected cytokine production also suggests a contribution of intracellular galectin-3 mediated interactions.
Overall, these studies indicate that depending on the galectin-variant or interaction partners cytosolic association with galectins provides ambivalent characteristics that can either dampen or elevate the immune response.
Intracellular binding partners of galectins in cancer
Many of the described roles of galectins in cancer can be assigned to interactions in the extracellular milieu. Nevertheless, some examples show that intracellular galectin interactions determine the fate of cancer progression. Cytosolic interaction of galectin-1 with H-Ras as mentioned above, stabilizes anchorage of the complex on the cytoplasmic membrane leaflet and further activates downstream oncogenic pathways together with the Raf effector [112](Fig. 2). This oncogenic role of galectin-1 corresponds to the poor survival rate of patients with a high expression pattern of this galectin subtype in gastric cancer [113], lung cancer [114], renal cancer [115], hepatocellular carcinoma [116] and ovarian cancer [117]. Moreover, in the cytosol and the nucleus galectin-1 colocalizes with the glucose-regulated protein 78 (GRP78), most likely the cytosolic variant of this ER-chaperone [113](Fig. 2). GRP78, which is also known as BIP, is overexpressed in various cancer cells and contributes to tumour cell survival [118]. Molecular details of this interaction are only vaguely understood. Clues might come from the observation that GRP78 is closely related to and promotes epithelial–mesenchymal transition (EMT) in cancer cells [119], which is also affected by protocadherin-24 (PCDH24), another galectin-1 interacting polypeptide [120, 121].
In analogy to galectin-1, galectin-3 interaction with K-Ras mediates membrane localization, stabilizes the GTPase in its active state and presents composite interfaces to facilitate Raf binding [49, 122, 45,46,47]. The multifaceted roles of galectin-3 in cancer progression have been summarized by Thijjsen et al. [123]. Of notice, galectin-3 itself is posttranslationally modified in distinct cancer tissues. In human breast cancer cells, the non-receptor tyrosine kinases c-Abl/Arg directly interact with and phosphorylate the N-terminal domain of cytosolic galectin-3 (Fig. 2). This phosphorylation occurs at tyrosine residues 79, 107, and 118, with Tyr-107 identified as the primary phosphorylation site [124, 125]. Moreover, galectin-3 can undergo cleavage, likely extracellularly, at this site by the chymotrypsin-like serine protease prostate-specific antigen (PSA) [126]. The phosphorylation at Tyr-107, mediated by c-Abl, inhibits this cleavage process. Consequently, unphosphorylated galectin-3 secreted by non-cancerous prostate cells is susceptible to cleavage by PSA, leading to the removal of the N-terminal domain. This cleavage event has the potential to reduce oligomerization and lattice formation by galectin-3 [127]. Interestingly, phosphorylation also seems to determine the intracellular fate of galectin-3 in cancer cells. If c-Abl/Arg kinases are absent, intracellular galectin-3 is recruited into lysosomes and degraded [125].
As mentioned before, the serine/threonine kinase CK1 phosphorylates galectin-3 at Ser-6 (Fig. 2). Apart from modulation of the subcellular lectin distribution between the nucleus and the cytosol, this phosphorylation is also required for tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis of breast cancer cells [128]. Phosphorylated galectin-3 activates a non-classical caspase activation cascade through induction of PTEN expression and inactivation of the PI3K/Akt survival pathway. The precise mechanisms responsible for galectin-3-mediated PTEN expression remain unclear at present. However, it appears that these mechanisms are facilitated by nuclear interactions of the lectin with transcription factors and/or heterogeneous nuclear ribonucleoproteins (hnRNPs). The possibility of an interaction between phosphorylated galectin-3 and PTEN cannot be ruled out either.
A different site of action for galectin-3 are the WNT/β-catenin and STAT3 signalling pathways (Fig. 2). Binding of galectin-3 to the transcription factor STAT3 enhances its Tyr-705 phosphorylation, leading to its nuclear translocation and transcriptional activation in gastric cancer tissues [129]. Furthermore, galectin-3 expression is elevated by WNT/β-catenin signalling. The lectin also interacts with β-catenin and promotes its translocation into the nucleus for activation of target genes. This study suggests that galectin-3 mediates the crosstalk between WNT/β-catenin and STAT3 signalling in tumour progression. Consequently, increased expression and co-localization of β-catenin, pSTAT3, and galectin-3 in patients with advanced gastric cancer correlates with a poorer prognosis.
Intracellular interaction of galectin-13 with the transcription factor HOXA1 does not seem to be exclusively important for embryonic development but also appears to be involved in cancer progression, since upregulation of HOXA1 is associated with a poor prognosis in patients with breast cancer or hepatocellular carcinoma [130, 131].
For galectin-7, interaction with mitochondrial Bcl-2 is implicated in the regulation of apoptotic cell death of colon carcinoma cells [132]. Bcl-2, which resides in the outer mitochondrial membrane oriented towards the cytosol, directly interacts with galectin-7 and recruits the lectin independently of carbohydrate recognition to mitochondria (Fig. 2). Mitochondrial localization and intracellular functioning of galectin-7 in apoptosis had been previously reported [133]. Upon binding to Bcl-2 galectin-7 may act as a pro-apoptotic protein that assists in initiation of the apoptotic cascade in cancer cells.
Concluding remarks and future perspectives
The past three decades have witnessed a significant increase in the identified intracellular interaction partners of galectins. Obviously, the focus of many of these studies concentrates on prototype galectin-1 and chimeric galectin-3. This is most likely due to the ubiquitous expression of these two galectins in many distinct tissues. Nevertheless, even if we narrow down our attention on these two galectins, many of the cellular interactions and their consequences still require further elucidation, especially on the molecular level. With respect to the remaining members of the galectin family extensive analysis of their intracellular roles should not be neglected. Even if just a defined number of examples is discussed in this review, it is self-evident that each member of this family possesses its individual pattern of cytosolic binding partners that await to be identified. We will have to fully characterize this network and identify the molecular details to develop strategies to cure diseases associated with galectin functions.
Data availability
Data sharing is not applicable as no datasets were generated or analysed for this review article.
References
Reily C, Stewart TJ, Renfrow MB, Novak J (2019) Glycosylation in health and disease. Nat Rev Nephrol 15(6):346–366. https://doi.org/10.1038/s41581-019-0129-4
Barondes SH, Cooper DN, Gitt MA, Leffler H (1994) Galectins. Structure and function of a large family of animal lectins. JBiolChem 269(33):20807–20810
Teichberg VI, Silman I, Beitsch DD, Resheff G (1975) A beta-D-galactoside binding protein from electric organ tissue of Electrophorus electricus. Proc Natl Acad Sci USA 72(4):1383–1387. https://doi.org/10.1073/pnas.72.4.1383
Lobsanov YD, Gitt MA, Leffler H, Barondes SH, Rini JM (1993) X-ray crystal structure of the human dimeric S-Lac lectin, L-14-II, in complex with lactose at 2.9-A resolution. J Biol Chem 268(36):27034–27038. https://doi.org/10.2210/pdb1hlc/pdb
Houzelstein D, Goncalves IR, Fadden AJ, Sidhu SS, Cooper DN, Drickamer K, Leffler H, Poirier F (2004) Phylogenetic analysis of the vertebrate galectin family. Mol Biol Evol 21(7):1177–1187. https://doi.org/10.1093/molbev/msh082
Chen L, Li F (2013) Structural analysis of the evolutionary origins of influenza virus hemagglutinin and other viral lectins. J Virol 87(7):4118–4120. https://doi.org/10.1128/JVI.03476-12
Stowell SR, Dias-Baruffi M, Penttila L, Renkonen O, Nyame AK, Cummings RD (2004) Human galectin-1 recognition of poly-N-acetyllactosamine and chimeric polysaccharides. Glycobiology 14(2):157–167. https://doi.org/10.1093/glycob/cwh018
Sindrewicz P, Li X, Yates EA, Turnbull JE, Lian LY, Yu LG (2019) Intrinsic tryptophan fluorescence spectroscopy reliably determines galectin-ligand interactions. Sci Rep 9(1):11851. https://doi.org/10.1038/s41598-019-47658-8
Hsieh TJ, Lin HY, Tu Z, Huang BS, Wu SC, Lin CH (2015) Structural basis underlying the binding preference of human Galectins-1, -3 and – 7 for Galbeta1-3/4GlcNAc. PLoS ONE 10(5):e0125946. https://doi.org/10.1371/journal.pone.0125946
Hirabayashi J, Hashidate T, Arata Y, Nishi N, Nakamura T, Hirashima M, Urashima T, Oka T, Futai M, Muller WE, Yagi F, Kasai K (2002) Oligosaccharide specificity of galectins: a search by frontal affinity chromatography. BiochimBiophysActa 1572(2–3):232–254 doi:S0304416502003112 [pii]
Marino KV, Cagnoni AJ, Croci DO, Rabinovich GA (2023) Targeting galectin-driven regulatory circuits in cancer and fibrosis. Nat Rev Drug Discov 22(4):295–316. https://doi.org/10.1038/s41573-023-00636-2
Di LS, Sundblad V, Cerliani JP, Guardia CM, Estrin DA, Vasta GR, Rabinovich GA (2011) When galectins recognize glycans: from biochemistry to physiology and back again. Biochemistry 50(37):7842–7857. https://doi.org/10.1021/bi201121m[doi]
Visegrady B, Than NG, Kilar F, Sumegi B, Than GN, Bohn H (2001) Homology modelling and molecular dynamics studies of human placental tissue protein 13 (galectin-13). Protein Eng 14(11):875–880. https://doi.org/10.1093/protein/14.11.875
Than NG, Romero R, Goodman M, Weckle A, Xing J, Dong Z, Xu Y, Tarquini F, Szilagyi A, Gal P, Hou Z, Tarca AL, Kim CJ, Kim JS, Haidarian S, Uddin M, Bohn H, Benirschke K, Santolaya-Forgas J, Grossman LI, Erez O, Hassan SS, Zavodszky P, Papp Z, Wildman DE (2009) A primate subfamily of galectins expressed at the maternal-fetal interface that promote immune cell death. Proc Natl Acad Sci USA 106(24):9731–9736. https://doi.org/10.1073/pnas.0903568106
Davidson PJ, Li SY, Lohse AG, Vandergaast R, Verde E, Pearson A, Patterson RJ, Wang JL, Arnoys EJ (2006) Transport of galectin-3 between the nucleus and cytoplasm. I. conditions and signals for nuclear import. Glycobiology 16(7):602–611. https://doi.org/10.1093/glycob/cwj088
Nakahara S, Hogan V, Inohara H, Raz A (2006) Importin-mediated nuclear translocation of galectin-3. J Biol Chem 281(51):39649–39659. https://doi.org/10.1074/jbc.M608069200
Tsay YG, Lin NY, Voss PG, Patterson RJ, Wang JL (1999) Export of galectin-3 from nuclei of digitonin-permeabilized mouse 3T3 fibroblasts. Exp Cell Res 252(2):250–261. https://doi.org/10.1006/excr.1999.4643
Takenaka Y, Fukumori T, Yoshii T, Oka N, Inohara H, Kim HR, Bresalier RS, Raz A (2004) Nuclear export of phosphorylated galectin-3 regulates its antiapoptotic activity in response to chemotherapeutic drugs. Mol Cell Biol 24(10):4395–4406. https://doi.org/10.1128/MCB.24.10.4395-4406.2004
Huflejt ME, Turck CW, Lindstedt R, Barondes SH, Leffler H (1993) L-29, a soluble lactose-binding lectin, is phosphorylated on serine 6 and serine 12 in vivo and by casein kinase I. J Biol Chem 268(35):26712–26718
Balan V, Nangia-Makker P, Schwartz AG, Jung YS, Tait L, Hogan V, Raz T, Wang Y, Yang ZQ, Wu GS, Guo Y, Li H, Abrams J, Couch FJ, Lingle WL, Lloyd RV, Ethier SP, Tainsky MA, Raz A (2008) Racial disparity in breast cancer and functional germ line mutation in galectin-3 (rs4644): a pilot study. Cancer Res 68(24):10045–10050. https://doi.org/10.1158/0008-5472.CAN-08-3224
Kim SJ, Shin JY, Cheong TC, Choi IJ, Lee YS, Park SH, Chun KH (2011) Galectin-3 germline variant at position 191 enhances nuclear accumulation and activation of beta-catenin in gastric cancer. Clin Exp Metastasis 28(8):743–750. https://doi.org/10.1007/s10585-011-9406-8
Voss PG, Wang JL (2023) Liquid-liquid phase separation: Galectin-3 in nuclear speckles and ribonucleoprotein complexes. Exp Cell Res 427(1):113571. https://doi.org/10.1016/j.yexcr.2023.113571
Fritsch K, Mernberger M, Nist A, Stiewe T, Brehm A, Jacob R (2016) Galectin-3 interacts with components of the nuclear ribonucleoprotein complex. BMC Cancer 16:502. https://doi.org/10.1186/s12885-016-2546-0
Park JW, Voss PG, Grabski S, Wang JL, Patterson RJ (2001) Association of galectin-1 and galectin-3 with Gemin4 in complexes containing the SMN protein. Nucleic Acids Res 29(17):3595–3602. https://doi.org/10.1093/nar/29.17.3595
Haudek KC, Voss PG, Locascio LE, Wang JL, Patterson RJ (2009) A mechanism for incorporation of galectin-3 into the spliceosome through its association with U1 snRNP. Biochemistry 48(32):7705–7712. https://doi.org/10.1021/bi900071b
Dagher SF, Wang JL, Patterson RJ (1995) Identification of galectin-3 as a factor in pre-mRNA splicing. Proc Natl Acad Sci USA 92(4):1213–1217
Vyakarnam A, Dagher SF, Wang JL, Patterson RJ (1997) Evidence for a role for galectin-1 in pre-mRNA splicing. Mol Cell Biol 17(8):4730–4737
Wang W, Park JW, Wang JL, Patterson RJ (2006) Immunoprecipitation of spliceosomal RNAs by antisera to galectin-1 and galectin-3. Nucleic Acids Res 34(18):5166–5174. https://doi.org/10.1093/nar/gkl673
Wei J, Wu Y, Sun Y, Chen D (2023) Galectin-1 regulates RNA expression and alternative splicing of angiogenic genes in HUVECs. Front Biosci (Landmark Ed) 28(4):74. https://doi.org/10.31083/j.fbl2804074
Voss PG, Gray RM, Dickey SW, Wang W, Park JW, Kasai K, Hirabayashi J, Patterson RJ, Wang JL (2008) Dissociation of the carbohydrate-binding and splicing activities of galectin-1. Arch Biochem Biophys 478(1):18–25. https://doi.org/10.1016/j.abb.2008.07.003
Haudek KC, Voss PG, Wang JL, Patterson RJ (2016) A 10S galectin-3-U1 snRNP complex assembles into active spliceosomes. Nucleic Acids Res 44(13):6391–6397. https://doi.org/10.1093/nar/gkw303
Coppin L, Vincent A, Frenois F, Duchene B, Lahdaoui F, Stechly L, Renaud F, Villenet C, Van Seuningen I, Leteurtre E, Dion J, Grandjean C, Poirier F, Figeac M, Delacour D, Porchet N, Pigny P (2017) Galectin-3 is a non-classic RNA binding protein that stabilizes the mucin MUC4 mRNA in the cytoplasm of cancer cells. Sci Rep 7:43927. https://doi.org/10.1038/srep43927
Bafna S, Kaur S, Batra SK (2010) Membrane-bound mucins: the mechanistic basis for alterations in the growth and survival of cancer cells. Oncogene 29(20):2893–2904. https://doi.org/10.1038/onc.2010.87
Song S, Byrd JC, Mazurek N, Liu K, Koo JS, Bresalier RS (2005) Galectin-3 modulates MUC2 mucin expression in human colon cancer cells at the level of transcription via AP-1 activation. Gastroenterology 129(5):1581–1591. https://doi.org/10.1053/j.gastro.2005.09.002
Kim SJ, Wang YG, Lee HW, Kang HG, La SH, Choi IJ, Irimura T, Ro JY, Bresalier RS, Chun KH (2014) Up-regulation of neogenin-1 increases cell proliferation and motility in gastric cancer. Oncotarget 5(10):3386–3398. https://doi.org/10.18632/oncotarget.1960
Jia W, Kong L, Kidoya H, Naito H, Muramatsu F, Hayashi Y, Hsieh HY, Yamakawa D, Hsu DK, Liu FT, Takakura N (2021) Indispensable role of Galectin-3 in promoting quiescence of hematopoietic stem cells. Nat Commun 12(1):2118. https://doi.org/10.1038/s41467-021-22346-2
Yang T, Yao Y, Wang X, Li Y, Si Y, Li X, Ayala GJ, Wang Y, Mayo KH, Tai G, Zhou Y, Su J (2020) Galectin-13/placental protein 13: redox-active disulfides as switches for regulating structure, function and cellular distribution. Glycobiology 30(2):120–129. https://doi.org/10.1093/glycob/cwz081
Lambert B, Vandeputte J, Remacle S, Bergiers I, Simonis N, Twizere JC, Vidal M, Rezsohazy R (2012) Protein interactions of the transcription factor Hoxa1. BMC Dev Biol 12:29. https://doi.org/10.1186/1471-213X-12-29
Than NG, Pick E, Bellyei S, Szigeti A, Burger O, Berente Z, Janaky T, Boronkai A, Kliman H, Meiri H, Bohn H, Than GN, Sumegi B (2004) Functional analyses of placental protein 13/galectin-13. Eur J Biochem 271(6):1065–1078. https://doi.org/10.1111/j.1432-1033.2004.04004.x
Rolland T, Tasan M, Charloteaux B, Pevzner SJ, Zhong Q, Sahni N, Yi S, Lemmens I, Fontanillo C, Mosca R, Kamburov A, Ghiassian SD, Yang X, Ghamsari L, Balcha D, Begg BE, Braun P, Brehme M, Broly MP, Carvunis AR, Convery-Zupan D, Corominas R, Coulombe-Huntington J, Dann E, Dreze M, Dricot A, Fan C, Franzosa E, Gebreab F, Gutierrez BJ, Hardy MF, Jin M, Kang S, Kiros R, Lin GN, Luck K, MacWilliams A, Menche J, Murray RR, Palagi A, Poulin MM, Rambout X, Rasla J, Reichert P, Romero V, Ruyssinck E, Sahalie JM, Scholz A, Shah AA, Sharma A, Shen Y, Spirohn K, Tam S, Tejeda AO, Wanamaker SA, Twizere JC, Vega K, Walsh J, Cusick ME, Xia Y, Barabasi AL, Iakoucheva LM, Aloy P, De Las Rivas J, Tavernier J, Calderwood MA, Hill DE, Hao T, Roth FP, Vidal M (2014) A proteome-scale map of the human interactome network. Cell 159(5):1212–1226. https://doi.org/10.1016/j.cell.2014.10.050
Si Y, Yao Y, Jaramillo Ayala G, Li X, Han Q, Zhang W, Xu X, Tai G, Mayo KH, Zhou Y, Su J (2021) Human galectin-16 has a pseudo ligand binding site and plays a role in regulating c-Rel-mediated lymphocyte activity. Biochim Biophys Acta Gen Subj 1865(1):129755. https://doi.org/10.1016/j.bbagen.2020.129755
Si Y, Li Y, Yang T, Li X, Ayala GJ, Mayo KH, Tai G, Su J, Zhou Y (2021) Structure-function studies of galectin-14, an important effector molecule in embryology. FEBS J 288(3):1041–1055. https://doi.org/10.1111/febs.15441
Belanis L, Plowman SJ, Rotblat B, Hancock JF, Kloog Y (2008) Galectin-1 is a novel structural component and a major regulator of h-ras nanoclusters. Mol Biol Cell 19(4):1404–1414. https://doi.org/10.1091/mbc.e07-10-1053
Paz A, Haklai R, Elad-Sfadia G, Ballan E, Kloog Y (2001) Galectin-1 binds oncogenic H-Ras to mediate ras membrane anchorage and cell transformation. Oncogene 20(51):7486–7493. https://doi.org/10.1038/sj.onc.1204950
Shalom-Feuerstein R, Plowman SJ, Rotblat B, Ariotti N, Tian T, Hancock JF, Kloog Y (2008) K-ras nanoclustering is subverted by overexpression of the scaffold protein galectin-3. Cancer Res 68(16):6608–6616. https://doi.org/10.1158/0008-5472.CAN-08-1117
Elad-Sfadia G, Haklai R, Balan E, Kloog Y (2004) Galectin-3 augments K-Ras activation and triggers a ras signal that attenuates ERK but not phosphoinositide 3-kinase activity. J Biol Chem 279(33):34922–34930. https://doi.org/10.1074/jbc.M312697200
Ashery U, Yizhar O, Rotblat B, Elad-Sfadia G, Barkan B, Haklai R, Kloog Y (2006) Spatiotemporal organization of Ras signaling: rasosomes and the galectin switch. Cell Mol Neurobiol 26(4–6):471–495. https://doi.org/10.1007/s10571-006-9059-3
Blazevits O, Mideksa YG, Solman M, Ligabue A, Ariotti N, Nakhaeizadeh H, Fansa EK, Papageorgiou AC, Wittinghofer A, Ahmadian MR, Abankwa D (2016) Galectin-1 dimers can scaffold raf-effectors to increase H-ras nanoclustering. Sci Rep 6:24165. https://doi.org/10.1038/srep24165
Mysore VP, Zhou ZW, Ambrogio C, Li L, Kapp JN, Lu C, Wang Q, Tucker MR, Okoro JJ, Nagy-Davidescu G, Bai X, Pluckthun A, Janne PA, Westover KD, Shan Y, Shaw DE (2021) A structural model of a ras-raf signalosome. Nat Struct Mol Biol 28(10):847–857. https://doi.org/10.1038/s41594-021-00667-6
Lukyanov P, Furtak V, Ochieng J (2005) Galectin-3 interacts with membrane lipids and penetrates the lipid bilayer. Biochem Biophys Res Commun 338(2):1031–1036. https://doi.org/10.1016/j.bbrc.2005.10.033
Magescas J, Sengmanivong L, Viau A, Mayeux A, Dang T, Burtin M, Nilsson UJ, Leffler H, Poirier F, Terzi F, Delacour D (2017) Spindle Pole cohesion requires glycosylation-mediated localization of NuMA. Sci Rep 7(1):1474. https://doi.org/10.1038/s41598-017-01614-6
Koch A, Poirier F, Jacob R, Delacour D (2010) Galectin-3, a novel centrosome-associated protein, required for epithelial morphogenesis. MolBiolCell 21(2):219–231 doi:E09-03-0193 [pii];10.1091/mbc.E09-03-0193 [doi]
Clare DK, Magescas J, Piolot T, Dumoux M, Vesque C, Pichard E, Dang T, Duvauchelle B, Poirier F, Delacour D (2014) Basal foot MTOC organizes pillar MTs required for coordination of beating cilia. Nat Commun 5:4888. https://doi.org/10.1038/ncomms5888
Mathew MP, Abramowitz LK, Donaldson JG, Hanover JA (2022) Nutrient-responsive O-GlcNAcylation dynamically modulates the secretion of glycan-binding protein galectin 3. J Biol Chem 298(3):101743. https://doi.org/10.1016/j.jbc.2022.101743
Ideo H, Hoshi I, Yamashita K, Sakamoto M (2013) Phosphorylation and externalization of galectin-4 is controlled by Src family kinases. Glycobiology 23(12):1452–1462. https://doi.org/10.1093/glycob/cwt073
Hurley JH, Hanson PI (2010) Membrane budding and scission by the ESCRT machinery: it’s all in the neck. Nat Rev Mol Cell Biol 11(8):556–566. https://doi.org/10.1038/nrm2937
Banfer S, Schneider D, Dewes J, Strauss MT, Freibert SA, Heimerl T, Maier UG, Elsasser HP, Jungmann R, Jacob R (2018) Molecular mechanism to recruit galectin-3 into multivesicular bodies for polarized exosomal secretion. Proc Natl Acad Sci USA 115(19):E4396–E4405. https://doi.org/10.1073/pnas.1718921115
Banfer S, Kutscher S, Fleck F, Dienst M, Preusser C, Pogge von Strandmann E, Jacob R (2022) Late domain dependent E-cadherin recruitment into extracellular vesicles. Front Cell Dev Biol 10:878620. https://doi.org/10.3389/fcell.2022.878620
Fei F, Joo EJ, Tarighat SS, Schiffer I, Paz H, Fabbri M, Abdel-Azim H, Groffen J, Heisterkamp N (2015) B-cell precursor acute lymphoblastic leukemia and stromal cells communicate through Galectin-3. Oncotarget 6(13):11378–11394. https://doi.org/10.18632/oncotarget.3409
Maybruck BT, Pfannenstiel LW, Diaz-Montero M, Gastman BR (2017) Tumor-derived exosomes induce CD8(+) T cell suppressors. J Immunother Cancer 5(1):65. https://doi.org/10.1186/s40425-017-0269-7
Ayechu-Muruzabal V, de Boer M, Blokhuis B, Berends AJ, Garssen J, Kraneveld AD, Van’t Land B, Willemsen LEM (2022) Epithelial-derived galectin-9 containing exosomes contribute to the immunomodulatory effects promoted by 2’-fucosyllactose and short-chain galacto- and long-chain fructo-oligosaccharides. Front Immunol 13:1026031. https://doi.org/10.3389/fimmu.2022.1026031
Anand PK (2010) Exosomal membrane molecules are potent immune response modulators. Commun Integr Biol 3(5):405–408. https://doi.org/10.4161/cib.3.5.12474
Than NG, Abdul Rahman O, Magenheim R, Nagy B, Fule T, Hargitai B, Sammar M, Hupuczi P, Tarca AL, Szabo G, Kovalszky I, Meiri H, Sziller I, Rigo J Jr., Romero R, Papp Z (2008) Placental protein 13 (galectin-13) has decreased placental expression but increased shedding and maternal serum concentrations in patients presenting with preterm pre-eclampsia and HELLP syndrome. Virchows Arch 453(4):387–400. https://doi.org/10.1007/s00428-008-0658-x
Fukuchi M, Kamide Y, Ueki S, Miyabe Y, Konno Y, Oka N, Takeuchi H, Koyota S, Hirokawa M, Yamada T, Melo RCN, Weller PF, Taniguchi M (2021) Eosinophil ETosis-Mediated release of Galectin-10 in Eosinophilic Granulomatosis with Polyangiitis. Arthritis Rheumatol 73(9):1683–1693. https://doi.org/10.1002/art.41727
Delacour D, Cramm-Behrens CI, Drobecq H, Le Bivic A, Naim HY, Jacob R (2006) Requirement for galectin-3 in apical protein sorting. CurrBiol 16(4):408–414
Delacour D, Gouyer V, Zanetta JP, Drobecq H, Leteurtre E, Grard G, Moreau-Hannedouche O, Maes E, Pons A, Andre S, Le Bivic A, Gabius HJ, Manninen A, Simons K, Huet G (2005) Galectin-4 and sulfatides in apical membrane trafficking in enterocyte-like cells. JCell Biol 169(3):491–501
Straube T, von Honig MT, Greb E, Schneider C, Jacob D R (2013) PH-dependent recycling of galectin-3 at the apical membrane of epithelial cells. Traffic 14(9):1014–1027. https://doi.org/10.1111/tra.12086[doi]
Mishra R, Grzybek M, Niki T, Hirashima M, Simons K (2010) Galectin-9 trafficking regulates apical-basal polarity in Madin-Darby canine kidney epithelial cells. ProcNatlAcadSciUSA 107(41):17633–17638 doi:1012424107 [pii]; https://doi.org/10.1073/pnas.1012424107 [doi]
Stechly L, Morelle W, Dessein AF, Andre S, Grard G, Trinel D, Dejonghe MJ, Leteurtre E, Drobecq H, Trugnan G, Gabius HJ, Huet G (2009) Galectin-4-regulated delivery of glycoproteins to the brush border membrane of enterocyte-like cells. Traffic 10(4):438–450. https://doi.org/10.1111/j.1600-0854.2009.00882.x
Delacour D, Greb C, Koch A, Salomonsson E, Leffler H, Le Bivic A, Jacob R (2007) Apical sorting by galectin-3-Dependent glycoprotein clustering. Traffic 8(4):379–388
Laiko M, Murtazina R, Malyukova I, Zhu C, Boedeker EC, Gutsal O, O’Malley R, Cole RN, Tarr PI, Murray KF, Kane A, Donowitz M, Kovbasnjuk O (2010) Shiga toxin 1 interaction with enterocytes causes apical protein mistargeting through the depletion of intracellular galectin-3. Exp Cell Res 316(4):657–666. https://doi.org/10.1016/j.yexcr.2009.09.002
Delacour D, Koch A, Ackermann W, Eude-Le Parco I, Elsasser HP, Poirier F, Jacob R (2008) Loss of galectin-3 impairs membrane polarisation of mouse enterocytes in vivo. JCell Sci 121(Pt 4):458–465
Honig E, Ringer K, Dewes J, von Mach T, Kamm N, Kreitzer G, Jacob R (2018) Galectin-3 modulates the polarized surface delivery of beta1-integrin in epithelial cells. J Cell Sci 131(11). https://doi.org/10.1242/jcs.213199
Schneider D, Greb C, Koch A, Straube T, Elli A, Delacour D, Jacob R (2010) Trafficking of galectin-3 through endosomal organelles of polarized and non-polarized cells. EurJCell Biol 89(11):788–798. https://doi.org/10.1016/j.ejcb.2010.07.001
Roman-Fernandez A, Mansour MA, Kugeratski FG, Anand J, Sandilands E, Galbraith L, Rakovic K, Freckmann EC, Cumming EM, Park J, Nikolatou K, Lilla S, Shaw R, Strachan D, Mason S, Patel R, McGarry L, Katoch A, Campbell KJ, Nixon C, Miller CJ, Leung HY, Le Quesne J, Norman JC, Zanivan S, Blyth K, Bryant DM (2023) Spatial regulation of the glycocalyx component podocalyxin is a switch for prometastatic function. Sci Adv 9(5):eabq1858. https://doi.org/10.1126/sciadv.abq1858
Lakshminarayan R, Wunder C, Becken U, Howes MT, Benzing C, Arumugam S, Sales S, Ariotti N, Chambon V, Lamaze C, Loew D, Shevchenko A, Gaus K, Parton RG, Johannes L (2014) Galectin-3 drives glycosphingolipid-dependent biogenesis of clathrin-independent carriers. Nat Cell Biol 16(6):595–606. https://doi.org/10.1038/ncb2970
Ivashenka A, Wunder C, Chambon V, Sandhoff R, Jennemann R, Dransart E, Podsypanina K, Lombard B, Loew D, Lamaze C, Poirier F, Grone HJ, Johannes L, Shafaq-Zadah M (2021) Glycolipid-dependent and lectin-driven transcytosis in mouse enterocytes. Commun Biol 4(1):173. https://doi.org/10.1038/s42003-021-01693-2
von Mach T, Carlsson MC, Straube T, Nilsson U, Leffler H, Jacob R (2014) Ligand binding and complex formation of galectin-3 is modulated by pH variations. Biochem J 457(1):107–115. https://doi.org/10.1042/BJ20130933
Perez Bay AE, Schreiner R, Benedicto I, Rodriguez-Boulan EJ (2014) Galectin-4-mediated transcytosis of transferrin receptor. J Cell Sci 127(Pt 20):4457–4469. https://doi.org/10.1242/jcs.153437
Sudhakar JN, Lu HH, Chiang HY, Suen CS, Hwang MJ, Wu SY, Shen CN, Chang YM, Li FA, Liu FT, Shui JW (2020) Lumenal galectin-9-Lamp2 interaction regulates lysosome and autophagy to prevent pathogenesis in the intestine and pancreas. Nat Commun 11(1):4286. https://doi.org/10.1038/s41467-020-18102-7
Kojima R, Ohno T, Iikura M, Niki T, Hirashima M, Iwaya K, Tsuda H, Nonoyama S, Matsuda A, Saito H, Matsumoto K, Nakae S (2014) Galectin-9 enhances cytokine secretion, but suppresses survival and degranulation, in human mast cell line. PLoS ONE 9(1):e86106. https://doi.org/10.1371/journal.pone.0086106
Lv R, Bao Q, Li Y (2017) Regulation of M1–type and M2–type macrophage polarization in RAW264.7 cells by Galectin–9. Mol Med Rep 16(6):9111–9119. https://doi.org/10.3892/mmr.2017.7719
Santalla Mendez R, Rodgers Furones A, Classens R, Fedorova K, Haverdil M, Canela Capdevila M, van Duffelen A, Spruijt CG, Vermeulen M, Ter Beest M, van Spriel AB, Querol Cano L (2023) Galectin-9 interacts with Vamp-3 to regulate cytokine secretion in dendritic cells. Cell Mol Life Sci 80(10):306. https://doi.org/10.1007/s00018-023-04954-x
Klose M, Salloum JE, Gonschior H, Linder S (2019) SNX3 drives maturation of Borrelia phagosomes by forming a hub for PI(3)P, Rab5a, and galectin-9. J Cell Biol 218(9):3039–3059. https://doi.org/10.1083/jcb.201812106
Grozdanovic MM, Doyle CB, Liu L, Maybruck BT, Kwatia MA, Thiyagarajan N, Acharya KR, Ackerman SJ (2020) Charcot-Leyden crystal protein/galectin-10 interacts with cationic ribonucleases and is required for eosinophil granulogenesis. J Allergy Clin Immunol 146(2):377–389e310. https://doi.org/10.1016/j.jaci.2020.01.013
Deretic V, Saitoh T, Akira S (2013) Autophagy in infection, inflammation and immunity. Nat Rev Immunol 13(10):722–737. https://doi.org/10.1038/nri3532
Paz I, Sachse M, Dupont N, Mounier J, Cederfur C, Enninga J, Leffler H, Poirier F, Prevost MC, Lafont F, Sansonetti P (2010) Galectin-3, a marker for vacuole lysis by invasive pathogens. Cell Microbiol 12(4):530–544. https://doi.org/10.1111/j.1462-5822.2009.01415.x
Thurston TL, Wandel MP, von Muhlinen N, Foeglein A, Randow F (2012) Galectin 8 targets damaged vesicles for autophagy to defend cells against bacterial invasion. Nature 482(7385):414–418. https://doi.org/10.1038/nature10744
Jia J, Abudu YP, Claude-Taupin A, Gu Y, Kumar S, Choi SW, Peters R, Mudd MH, Allers L, Salemi M, Phinney B, Johansen T, Deretic V (2018) Galectins Control mTOR in response to endomembrane damage. Mol Cell 70(1):120–135e128. https://doi.org/10.1016/j.molcel.2018.03.009
Eapen VV, Swarup S, Hoyer MJ, Paulo JA, Harper JW (2021) Quantitative proteomics reveals the selectivity of ubiquitin-binding autophagy receptors in the turnover of damaged lysosomes by lysophagy. Elife 10. https://doi.org/10.7554/eLife.72328
Jia J, Bissa B, Brecht L, Allers L, Choi SW, Gu Y, Zbinden M, Burge MR, Timmins G, Hallows K, Behrends C, Deretic V (2020) AMPK, a Regulator of Metabolism and Autophagy, is activated by Lysosomal Damage via a novel galectin-Directed Ubiquitin Signal Transduction System. Mol Cell 77(5):951–969e959. https://doi.org/10.1016/j.molcel.2019.12.028
Jia J, Claude-Taupin A, Gu Y, Choi SW, Peters R, Bissa B, Mudd MH, Allers L, Pallikkuth S, Lidke KA, Salemi M, Phinney B, Mari M, Reggiori F, Deretic V (2020) Galectin-3 coordinates a Cellular System for Lysosomal Repair and removal. Dev Cell 52(1):69–87e68. https://doi.org/10.1016/j.devcel.2019.10.025
Chen HY, Fermin A, Vardhana S, Weng IC, Lo KF, Chang EY, Maverakis E, Yang RY, Hsu DK, Dustin ML, Liu FT (2009) Galectin-3 negatively regulates TCR-mediated CD4 + T-cell activation at the immunological synapse. Proc Natl Acad Sci USA 106(34):14496–14501. https://doi.org/10.1073/pnas.0903497106
Wang SF, Tsao CH, Lin YT, Hsu DK, Chiang ML, Lo CH, Chien FC, Chen P, Arthur Chen YM, Chen HY, Liu FT (2014) Galectin-3 promotes HIV-1 budding via association with Alix and Gag p6. Glycobiology 24(11):1022–1035. https://doi.org/10.1093/glycob/cwu064
Radulovic M, Schink KO, Wenzel EM, Nahse V, Bongiovanni A, Lafont F, Stenmark H (2018) ESCRT-mediated lysosome repair precedes lysophagy and promotes cell survival. EMBO J 37(21). https://doi.org/10.15252/embj.201899753
Skowyra ML, Schlesinger PH, Naismith TV, Hanson PI (2018) Triggered recruitment of ESCRT machinery promotes endolysosomal repair. Science 360(6384). https://doi.org/10.1126/science.aar5078
Chauhan S, Kumar S, Jain A, Ponpuak M, Mudd MH, Kimura T, Choi SW, Peters R, Mandell M, Bruun JA, Johansen T, Deretic V (2016) TRIMs and Galectins Globally Cooperate and TRIM16 and Galectin-3 co-direct autophagy in Endomembrane Damage Homeostasis. Dev Cell 39(1):13–27. https://doi.org/10.1016/j.devcel.2016.08.003
Kimura T, Mandell M, Deretic V (2016) Precision autophagy directed by receptor regulators - emerging examples within the TRIM family. J Cell Sci 129(5):881–891. https://doi.org/10.1242/jcs.163758
Jia P, Tian T, Li Z, Wang Y, Lin Y, Zeng W, Ye Y, He M, Ni X, Pan J, Dong X, Huang J, Li CM, Guo D, Hou P (2023) CCDC50 promotes tumor growth through regulation of lysosome homeostasis. EMBO Rep 24(10):e56948. https://doi.org/10.15252/embr.202356948
Stillman BN, Hsu DK, Pang M, Brewer CF, Johnson P, Liu FT, Baum LG (2006) Galectin-3 and galectin-1 bind distinct cell surface glycoprotein receptors to induce T cell death. J Immunol 176(2):778–789. https://doi.org/10.4049/jimmunol.176.2.778
Yang RY, Hsu DK, Liu FT (1996) Expression of galectin-3 modulates T-cell growth and apoptosis. Proc Natl Acad Sci USA 93(13):6737–6742
Mohammadpour H, Tsuji T, MacDonald CR, Sarow JL, Rosenheck H, Daneshmandi S, Choi JE, Qiu J, Matsuzaki J, Witkiewicz AK, Attwood K, Blazar BR, Odunsi K, Repasky EA, McCarthy PL (2023) Galectin-3 expression in donor T cells reduces GvHD severity and lethality after allogeneic hematopoietic cell transplantation. Cell Rep 42(3):112250. https://doi.org/10.1016/j.celrep.2023.112250
Fukumori T, Oka N, Takenaka Y, Nangia-Makker P, Elsamman E, Kasai T, Shono M, Kanayama HO, Ellerhorst J, Lotan R, Raz A (2006) Galectin-3 regulates mitochondrial stability and antiapoptotic function in response to anticancer drug in prostate cancer. Cancer Res 66(6):3114–3119. https://doi.org/10.1158/0008-5472.CAN-05-3750
Kaur M, Kumar D, Butty V, Singh S, Esteban A, Fink GR, Ploegh HL, Sehrawat S (2018) Galectin-3 regulates gamma-Herpesvirus specific CD8 T cell immunity. iScience 9:101–119. https://doi.org/10.1016/j.isci.2018.10.013
Chen HY, Wu YF, Chou FC, Wu YH, Yeh LT, Lin KI, Liu FT, Sytwu HK (2020) Intracellular Galectin-9 enhances proximal TCR Signaling and Potentiates Autoimmune diseases. J Immunol 204(5):1158–1172. https://doi.org/10.4049/jimmunol.1901114
Ortner D, Grabher D, Hermann M, Kremmer E, Hofer S, Heufler C (2011) The adaptor protein Bam32 in human dendritic cells participates in the regulation of MHC class I-induced CD8 + T cell activation. J Immunol 187(8):3972–3978. https://doi.org/10.4049/jimmunol.1003072
Querol Cano L, Tagit O, Dolen Y, van Duffelen A, Dieltjes S, Buschow SI, Niki T, Hirashima M, Joosten B, van den Dries K, Cambi A, Figdor CG, van Spriel AB (2019) Intracellular Galectin-9 controls dendritic cell function by maintaining plasma membrane rigidity. iScience 22:240–255. https://doi.org/10.1016/j.isci.2019.11.019
Alves CM, Silva DA, Azzolini AE, Marzocchi-Machado CM, Carvalho JV, Pajuaba AC, Lucisano-Valim YM, Chammas R, Liu FT, Roque-Barreira MC, Mineo JR (2010) Galectin-3 plays a modulatory role in the life span and activation of murine neutrophils during early Toxoplasma gondii infection. Immunobiology 215(6):475–485. https://doi.org/10.1016/j.imbio.2009.08.001
Wu SY, Huang JH, Chen WY, Chan YC, Lin CH, Chen YC, Liu FT, Wu-Hsieh BA (2017) Cell intrinsic Galectin-3 attenuates Neutrophil ROS-Dependent Killing of Candida by modulating CR3 downstream Syk activation. Front Immunol 8:48. https://doi.org/10.3389/fimmu.2017.00048
Fermin Lee A, Chen HY, Wan L, Wu SY, Yu JS, Huang AC, Miaw SC, Hsu DK, Wu-Hsieh BA, Liu FT (2013) Galectin-3 modulates Th17 responses by regulating dendritic cell cytokines. Am J Pathol 183(4):1209–1222. https://doi.org/10.1016/j.ajpath.2013.06.017
Fermino ML, Dylon LS, Cecilio NT, Santos SN, Toscano MA, Dias-Baruffi M, Roque-Barreira MC, Rabinovich GA, Bernardes ES (2016) Lack of galectin-3 increases Jagged1/Notch activation in bone marrow-derived dendritic cells and promotes dysregulation of T helper cell polarization. Mol Immunol 76:22–34. https://doi.org/10.1016/j.molimm.2016.06.005
Li ZL, Prakash P, Buck M (2018) A tug of War maintains a dynamic protein-membrane complex: Molecular Dynamics simulations of C-Raf RBD-CRD bound to K-Ras4B at an Anionic membrane. ACS Cent Sci 4(2):298–305. https://doi.org/10.1021/acscentsci.7b00593
Zhang Q, Ali M, Wang Y, Sun QN, Zhu XD, Tang D, Wang W, Zhang CY, Zhou HH, Wang DR (2022) Galectin–1 binds GRP78 to promote the proliferation and metastasis of gastric cancer. Int J Oncol 61(5). https://doi.org/10.3892/ijo.2022.5431
Carlini MJ, Roitman P, Nunez M, Pallotta MG, Boggio G, Smith D, Salatino M, Joffe ED, Rabinovich GA, Puricelli LI (2014) Clinical relevance of galectin-1 expression in non-small cell lung cancer patients. Lung Cancer 84(1):73–78. https://doi.org/10.1016/j.lungcan.2014.01.016
Huang CS, Tang SJ, Chung LY, Yu CP, Ho JY, Cha TL, Hsieh CC, Wang HH, Sun GH, Sun KH (2014) Galectin-1 upregulates CXCR4 to promote tumor progression and poor outcome in kidney cancer. J Am Soc Nephrol 25(7):1486–1495. https://doi.org/10.1681/ASN.2013070773
Zhang PF, Li KS, Shen YH, Gao PT, Dong ZR, Cai JB, Zhang C, Huang XY, Tian MX, Hu ZQ, Gao DM, Fan J, Ke AW, Shi GM (2016) Galectin-1 induces hepatocellular carcinoma EMT and sorafenib resistance by activating FAK/PI3K/AKT signaling. Cell Death Dis 7(4):e2201. https://doi.org/10.1038/cddis.2015.324
Schulz H, Schmoeckel E, Kuhn C, Hofmann S, Mayr D, Mahner S, Jeschke U (2017) Galectins-1, -3, and – 7 are prognostic markers for Survival of Ovarian Cancer patients. Int J Mol Sci 18(6). https://doi.org/10.3390/ijms18061230
Ibrahim IM, Abdelmalek DH, Elfiky AA (2019) GRP78: a cell’s response to stress. Life Sci 226:156–163. https://doi.org/10.1016/j.lfs.2019.04.022
Sun LL, Chen CM, Zhang J, Wang J, Yang CZ, Lin LZ (2019) Glucose-regulated protein 78 signaling regulates Hypoxia-Induced epithelial-mesenchymal transition in A549 cells. Front Oncol 9:137. https://doi.org/10.3389/fonc.2019.00137
Ose R, Oharaa O, Nagase T (2012) Galectin-1 and Galectin-3 mediate protocadherin-24-Dependent membrane localization of beta-catenin in Colon cancer cell line HCT116. Curr Chem Genomics 6:18–26. https://doi.org/10.2174/1875397301206010018
Ose R, Yanagawa T, Ikeda S, Ohara O, Koga H (2009) PCDH24-induced contact inhibition involves downregulation of beta-catenin signaling. Mol Oncol 3(1):54–66. https://doi.org/10.1016/j.molonc.2008.10.005
Levy R, Biran A, Poirier F, Raz A, Kloog Y (2011) Galectin-3 mediates cross-talk between K-Ras and Let-7c tumor suppressor microRNA. PLoS ONE 6(11):e27490. https://doi.org/10.1371/journal.pone.0027490
Thijssen VL, Heusschen R, Caers J, Griffioen AW (2015) Galectin expression in cancer diagnosis and prognosis: a systematic review. Biochim Biophys Acta 1855(2):235–247. https://doi.org/10.1016/j.bbcan.2015.03.003
Balan V, Nangia-Makker P, Jung YS, Wang Y, Raz A (2010) Galectin-3: a novel substrate for c-Abl kinase. Biochim Biophys Acta 1803(10):1198–1205. https://doi.org/10.1016/j.bbamcr.2010.06.007
Li X, Ma Q, Wang J, Liu X, Yang Y, Zhao H, Wang Y, Jin Y, Zeng J, Li J, Song L, Li X, Li P, Qian X, Cao C (2010) c-Abl and arg tyrosine kinases regulate lysosomal degradation of the oncoprotein Galectin-3. Cell Death Differ 17(8):1277–1287. https://doi.org/10.1038/cdd.2010.8
Balan V, Nangia-Makker P, Kho DH, Wang Y, Raz A (2012) Tyrosine-phosphorylated galectin-3 protein is resistant to prostate-specific antigen (PSA) cleavage. J Biol Chem 287(8):5192–5198. https://doi.org/10.1074/jbc.C111.331686
Nabi IR, Shankar J, Dennis JW (2015) The galectin lattice at a glance. J Cell Sci 128(13):2213–2219. https://doi.org/10.1242/jcs.151159
Mazurek N, Sun YJ, Liu KF, Gilcrease MZ, Schober W, Nangia-Makker P, Raz A, Bresalier RS (2007) Phosphorylated galectin-3 mediates tumor necrosis factor-related apoptosis-inducing ligand signaling by regulating phosphatase and tensin homologue deleted on chromosome 10 in human breast carcinoma cells. J Biol Chem 282(29):21337–21348. https://doi.org/10.1074/jbc.M608810200
Kim SJ, Kang HG, Kim K, Kim H, Zetterberg F, Park YS, Cho HS, Hewitt SM, Chung JY, Nilsson UJ, Leffler H, Chun KH (2021) Crosstalk between WNT and STAT3 is mediated by galectin-3 in tumor progression. Gastric Cancer 24(5):1050–1062. https://doi.org/10.1007/s10120-021-01186-5
Liu J, Liu J, Lu X (2019) HOXA1 upregulation is associated with poor prognosis and tumor progression in breast cancer. Exp Ther Med 17(3):1896–1902. https://doi.org/10.3892/etm.2018.7145
Zha TZ, Hu BS, Yu HF, Tan YF, Zhang Y, Zhang K (2012) Overexpression of HOXA1 correlates with poor prognosis in patients with hepatocellular carcinoma. Tumour Biol 33(6):2125–2134. https://doi.org/10.1007/s13277-012-0472-6
Villeneuve C, Baricault L, Canelle L, Barboule N, Racca C, Monsarrat B, Magnaldo T, Larminat F (2011) Mitochondrial proteomic approach reveals galectin-7 as a novel BCL-2 binding protein in human cells. Mol Biol Cell 22(7):999–1013. https://doi.org/10.1091/mbc.E10-06-0534
Kuwabara I, Kuwabara Y, Yang RY, Schuler M, Green DR, Zuraw BL, Hsu DK, Liu FT (2002) Galectin-7 (PIG1) exhibits pro-apoptotic function through JNK activation and mitochondrial cytochrome c release. J Biol Chem 277(5):3487–3497. https://doi.org/10.1074/jbc.M109360200
Jia J, Abudu YP, Claude-Taupin A, Gu Y, Kumar S, Choi SW, Peters R, Mudd MH, Allers L, Salemi M, Phinney B, Johansen T, Deretic V (2019) Galectins control MTOR and AMPK in response to lysosomal damage to induce autophagy. Autophagy 15(1):169–171. https://doi.org/10.1080/15548627.2018.1505155
Funding
We are indebted to the countless scientists whose research provided the basis for this review. The authors did not receive support from any organization for the submitted work.
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
Ralf Jacob had the idea of this review and wrote the first draft of the manuscript. Figures were designed by Lena Gorek. The two authors critically revised the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jacob, R., Gorek, LS. Intracellular galectin interactions in health and disease. Semin Immunopathol 46, 4 (2024). https://doi.org/10.1007/s00281-024-01010-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00281-024-01010-z