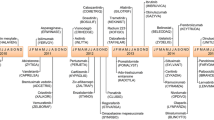
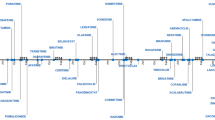
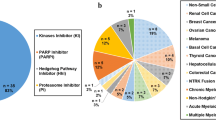
Abstract
During the last decade, the treatment for many cancer indications has evolved due to intensive clinical research into anti-tumor agents’ combination. In most instances, new combination treatments consist of an add-on to the standard of care (SOC), which then demonstrate a substantial gain in efficacy and no detrimental effect in tolerability. In the era of targeted therapies, for which maximum tolerated dose (MTD)-based dosing strategies are no longer applicable, early stage studies exploring new combinations are often conducted in the population of interest, expediting the collection of preliminary safety data, to be promptly expanded to collect preliminary efficacy data. Nevertheless, rule-based dose-finding studies are still a prevailing approach for early stage cancer, especially for chemotherapy (CT)-containing combinations. Pharmacokinetic (PK) assessments play a key role throughout the clinical development of drug combinations, informing potential PK interactions. But most importantly, they allow the development of innovative exposure–response (E–R) models aimed at exploring the contribution of each agent to the overall effect of the combination therapy. This review identifies 81 new drug combinations approved by the United States Food and Drug Administration (FDA) for hemato-oncology during the 2011–2021 period and summarizes the main design features of clinical trials and the role of PK assessments.





Similar content being viewed by others
References
Dameshek W, Necheles T, Finkle H, Allen D (1965) Therapy of Acute Leukemia, 1965. Blood 26(2):220
Sitki Copur M, Harrold L, Chu E (2021) Common Chemotherapy Regimens in Clinical Practice. In: Chu E, DeVita VT Physicians' Cancer Chemotherapy Drug Manual 2020. In: Jones and Bartlett Publishers I (ed). Boston, USA,
Department of Health and Human Services FDA (2013) Codevelopment of two or more new investigational drugs for use in combination. https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM236669.pdf. Accessed 8 July 2018
Administration USFaD (2016–2019) Hematology/Oncology (Cancer) Approvals & Safety Notifications. https://www.fda.gov/drugs/resources-information-approved-drugs/hematologyoncology-cancer-approvals-safety-notifications. Accessed January 5, 2020
Centerwatch FDA approved drugs for oncology. http://www.centerwatch.com/drug-information/fda-approved-drugs/therapeutic-area/12/oncology. Accessed 2 January, 2020
Administration USFaD Drugs@FDA: FDA approved drug products. . http://www.accessdata.fda.gov/scripts/cder/daf/index.cfm. Accessed 2 January, 2020
Department of Health and Human Services FaDA Drugs@FDA: FDA Approved Drug Products. https://www.accessdata.fda.gov/scripts/cder/daf/. Accessed July 18, 2018
Nottage M, Siu LL (2002) Principles of clinical trial design. J Clin Oncol 20(18 Suppl):42S-46S
Lexchin J, Graham J, Herder M, Jefferson T, Lemmens T (2021) Regulators, pivotal clinical trials, and drug regulation in the age of COVID-19. Int J Health Serv 51(1):5–13. https://doi.org/10.1177/0020731420979824
Lu D, Lu T, Stroh M, Graham RA, Agarwal P, Musib L, Li CC, Lum BL, Joshi A (2016) A survey of new oncology drug approvals in the USA from 2010 to 2015: a focus on optimal dose and related postmarketing activities. Cancer Chemother Pharmacol 77(3):459–476. https://doi.org/10.1007/s00280-015-2931-4
Mirza M, Avall-Lundqvist E, Birrer M (2019) Combination of niraparib and bevacizumab versus niraparib alone as treatment of recurrent platinum-sensitive ovarian cancer: a randomized controlled chemotherapy-free study — NSGOAVANOVA2/ENGOT-OV24. J Clin Oncol 37:Suppl: 5505
Vogl DT, Dingli D, Cornell RF, Huff CA, Jagannath S, Bhutani D, Zonder J, Baz R, Nooka A, Richter J, Cole C, Vij R, Jakubowiak A, Abonour R, Schiller G, Parker TL, Costa LJ, Kaminetzky D, Hoffman JE, Yee AJ, Chari A, Siegel D, Fonseca R, Van Wier S, Ahmann G, Lopez I, Kauffman M, Shacham S, Saint-Martin JR, Picklesimer CD, Choe-Juliak C, Stewart AK (2018) Selective inhibition of nuclear export with oral selinexor for treatment of relapsed or refractory multiple myeloma. J Clin Oncol 36(9):859–866. https://doi.org/10.1200/JCO.2017.75.5207
Leonard JP, Jung SH, Johnson J, Pitcher BN, Bartlett NL, Blum KA, Czuczman M, Giguere JK, Cheson BD (2015) Randomized trial of lenalidomide alone versus lenalidomide plus rituximab in patients with recurrent follicular lymphoma: CALGB 50401 (Alliance). J Clin Oncol 33(31):3635–3640. https://doi.org/10.1200/JCO.2014.59.9258
Baselga J, Semiglazov V, van Dam P, Manikhas A, Bellet M, Mayordomo J, Campone M, Kubista E, Greil R, Bianchi G, Steinseifer J, Molloy B, Tokaji E, Gardner H, Phillips P, Stumm M, Lane HA, Dixon JM, Jonat W, Rugo HS (2009) Phase II randomized study of neoadjuvant everolimus plus letrozole compared with placebo plus letrozole in patients with estrogen receptor-positive breast cancer. J Clin Oncol 27(16):2630–2637. https://doi.org/10.1200/JCO.2008.18.8391
Finn RS, Crown JP, Lang I, Boer K, Bondarenko IM, Kulyk SO, Ettl J, Patel R, Pinter T, Schmidt M, Shparyk Y, Thummala AR, Voytko NL, Fowst C, Huang X, Kim ST, Randolph S, Slamon DJ (2015) The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol 16(1):25–35. https://doi.org/10.1016/S1470-2045(14)71159-3
Skolnik JM, Barrett JS, Jayaraman B, Patel D, Adamson PC (2008) Shortening the timeline of pediatric phase I trials: the rolling six design. J Clin Oncol 26(2):190–195. https://doi.org/10.1200/JCO.2007.12.7712
Flaherty KT, Infante JR, Daud A, Gonzalez R, Kefford RF, Sosman J, Hamid O, Schuchter L, Cebon J, Ibrahim N, Kudchadkar R, Burris HA 3rd, Falchook G, Algazi A, Lewis K, Long GV, Puzanov I, Lebowitz P, Singh A, Little S, Sun P, Allred A, Ouellet D, Kim KB, Patel K, Weber J (2012) Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med 367(18):1694–1703. https://doi.org/10.1056/NEJMoa1210093
Tabernero J, Geel RV, Guren TK, Yaeger RD, Spreafico A, Faris JE, Yoshino T, Yamada Y, Kim TW, Bendell JC, Schuler MH, Lenz H, Eskens F, Desai J, Hochster HS, Avsar E, Demuth T, Sandor V, Elez E, Schellens JHM (2016) Phase 2 results: Encorafenib (ENCO) and cetuximab (CETUX) with or without alpelisib (ALP) in patients with advanced BRAF-mutant colorectal cancer (BRAFm CRC). J Clin Oncol 34(15_suppl):3544–3544. https://doi.org/10.1200/JCO.2016.34.15_suppl.3544
Lancet JE, Uy GL, Cortes JE, Newell LF, Lin TL, Ritchie EK, Stuart RK, Strickland SA, Hogge D, Solomon SR, Stone RM, Bixby DL, Kolitz JE, Schiller GJ, Wieduwilt MJ, Ryan DH, Hoering A, Banerjee K, Chiarella M, Louie AC, Medeiros BC (2018) CPX-351 (cytarabine and daunorubicin) liposome for injection versus conventional cytarabine plus daunorubicin in older patients with newly diagnosed secondary acute myeloid leukemia. J Clin Oncol 36(26):2684–2692. https://doi.org/10.1200/JCO.2017.77.6112
Garcia-Manero J, McCloskey J, Griffiths EA (2019) Pharmacokinetic Exposure Equivalence and Preliminary Efficacy and Safety from a Randomized Cross over Phase 3 Study (ASCERTAIN study) of an Oral Hypomethylating Agent ASTX727 (cedazuridine/decitabine) Compared to IV Decitabine. Blood 134(Supplement_1):846. https://doi.org/10.1182/blood-2019-122980
Dummer R, Ascierto PA, Gogas HJ, Arance A, Mandala M, Liszkay G, Garbe C, Schadendorf D, Krajsova I, Gutzmer R, Chiarion-Sileni V, Dutriaux C, de Groot JWB, Yamazaki N, Loquai C, Moutouh-de Parseval LA, Pickard MD, Sandor V, Robert C, Flaherty KT (2018) Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF-mutant melanoma (COLUMBUS): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 19(5):603–615. https://doi.org/10.1016/S1470-2045(18)30142-6
Chari A, Vogl DT, Gavriatopoulou M, Nooka AK, Yee AJ, Huff CA, Moreau P, Dingli D, Cole C, Lonial S, Dimopoulos M, Stewart AK, Richter J, Vij R, Tuchman S, Raab MS, Weisel KC, Delforge M, Cornell RF, Kaminetzky D, Hoffman JE, Costa LJ, Parker TL, Levy M, Schreder M, Meuleman N, Frenzel L, Mohty M, Choquet S, Schiller G, Comenzo RL, Engelhardt M, Illmer T, Vlummens P, Doyen C, Facon T, Karlin L, Perrot A, Podar K, Kauffman MG, Shacham S, Li L, Tang S, Picklesimer C, Saint-Martin JR, Crochiere M, Chang H, Parekh S, Landesman Y, Shah J, Richardson PG, Jagannath S (2019) Oral selinexor-dexamethasone for triple-class refractory multiple myeloma. N Engl J Med 381(8):727–738. https://doi.org/10.1056/NEJMoa1903455
Salles G, Duell J, Gonzalez Barca E, Tournilhac O, Jurczak W, Liberati AM, Nagy Z, Obr A, Gaidano G, Andre M, Kalakonda N, Dreyling M, Weirather J, Dirnberger-Hertweck M, Ambarkhane S, Fingerle-Rowson G, Maddocks K (2020) Tafasitamab plus lenalidomide in relapsed or refractory diffuse large B-cell lymphoma (L-MIND): a multicentre, prospective, single-arm, phase 2 study. Lancet Oncol 21(7):978–988. https://doi.org/10.1016/S1470-2045(20)30225-4
Chari A, Suvannasankha A, Fay JW, Arnulf B, Kaufman JL, Ifthikharuddin JJ, Weiss BM, Krishnan A, Lentzsch S, Comenzo R, Wang J, Nottage K, Chiu C, Khokhar NZ, Ahmadi T, Lonial S (2017) Daratumumab plus pomalidomide and dexamethasone in relapsed and/or refractory multiple myeloma. Blood 130(8):974–981. https://doi.org/10.1182/blood-2017-05-785246
Makker V, Taylor MH, Aghajanian C, Oaknin A, Mier J, Cohn AL, Romeo M, Bratos R, Brose MS, DiSimone C, Messing M, Stepan DE, Dutcus CE, Wu J, Schmidt EV, Orlowski R, Sachdev P, Shumaker R, Casado Herraez A (2020) Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer. J Clin Oncol 38(26):2981–2992. https://doi.org/10.1200/JCO.19.02627
Administration USFaD (2015) FARYDAK® (panobinostat) clinical pharmacology and biopharmaceutics review. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/205353Orig1s000TOC.cfm. Accessed October 16, 2021
(CHMP) CfMPfHU (2019) Tecentriq: EPAR - assessment report - variation
Bailly C, Thuru X, Quesnel B (2020) Combined cytotoxic chemotherapy and immunotherapy of cancer: modern times. NAR Cancer. https://doi.org/10.1093/narcan/zcaa002
Hungria VTM, Chiattone C, Pavlovsky M, Abenoza LM, Agreda GP, Armenta J, Arrais C, Avendaño-Flores O, Barroso F, Basquiera AL, Cao C, Cugliari MS, Enrico A, Foggliatto LM, Galvez KM, Gomez D, Gomez A, de Iracema D, Farias D, Lopez L, Mantilla WA, Martínez D, Mela MJ, Miguel CE, Ovilla R, Palmer L, Pavlovsky C, Ramos C, Remaggi G, Santucci R, Schusterschitz S, Sossa CL, Tuna-Aguilar E, Vela J, Santos T, de la Mora O, Machnicki G, Fernandez M, Barreyro P (2019) Epidemiology of hematologic malignancies in real-world settings: findings from the hemato-oncology latin america observational registry study. J Glob Oncol 5:1–19. https://doi.org/10.1200/jgo.19.00025
Bullock JM, Rahman A, Liu Q (2016) Lessons learned: dose selection of small molecule-targeted oncology drugs. Clin Cancer Res 22(11):2630–2638. https://doi.org/10.1158/1078-0432.CCR-15-2646
Jardim DL, Hess KR, Lorusso P, Kurzrock R, Hong DS (2014) Predictive value of phase I trials for safety in later trials and final approved dose: analysis of 61 approved cancer drugs. Clin Cancer Res 20(2):281–288. https://doi.org/10.1158/1078-0432.CCR-13-2103
Oncology Center of Excellence, Food and Drug Administration (2022) Project Optimus: Reforming the dose optimization and dose selection paradigm in oncology. https://www.fda.gov/about-fda/oncology-center-excellence/project-optimus. Accessed July 21, 2022
Iasonos A, O’Quigley J (2014) Adaptive dose-finding studies: a review of model-guided phase I clinical trials. J Clin Oncol 32(23):2505–2511. https://doi.org/10.1200/JCO.2013.54.6051
Riviere MK, Le Tourneau C, Paoletti X, Dubois F, Zohar S (2015) Designs of drug-combination phase I trials in oncology: a systematic review of the literature. Annals Oncol 26(4):669–674. https://doi.org/10.1093/annonc/mdu516
Wages NA, Ivanova A, Marchenko O (2016) Practical designs for Phase I combination studies in oncology. J Biopharm Stat 26(1):150–166. https://doi.org/10.1080/10543406.2015.1092029
Dumortier T, Looby M, Luttringer O, Heimann G, Klupp J, Junge G, Witte S, VanValen R, Stanski D (2015) Estimating the contribution of everolimus to immunosuppressive efficacy when combined with tacrolimus in liver transplantation: a model-based approach. Clin Pharmacol Ther 97(4):411–418. https://doi.org/10.1002/cpt.63
Zhao L, Hongshan L, Marathe A, Yu J, Rekić D, Mehrotra N, Sinha V, Wang Y (2016) New advancements in exposure-response analysis to inform regulatory decision making. In: Bonate PL, Howrad DR (eds) Pharmacokinetics in drug development. Springer International, Cham. https://doi.org/10.1007/978-3-319-39053-6_13
Wu K, House L, Ramirez J, Seminerio MJ, Ratain MJ (2013) Evaluation of utility of pharmacokinetic studies in phase I trials of two oncology drugs. Clin Cancer Res 19(21):6039–6043. https://doi.org/10.1158/1078-0432.CCR-13-0597
Author information
Authors and Affiliations
Contributions
SF and RL contributed to conception and design of the review. AS helped with the literature search and organization. AS, MU, and AC contributed to data extraction. SF wrote the manuscript. LPR drew the figures. BGA contributed with a critical assessment, and editorial review of the manuscript. AZ contributed with a critical assessment and expert review. All authors contributed to manuscript revision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fudio, S., Sellers, A., Pérez Ramos, L. et al. Anti-cancer drug combinations approved by US FDA from 2011 to 2021: main design features of clinical trials and role of pharmacokinetics. Cancer Chemother Pharmacol 90, 285–299 (2022). https://doi.org/10.1007/s00280-022-04467-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-022-04467-7