Abstract
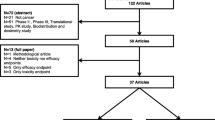
There has been increasing attention to dose optimization in the development of targeted oncology therapeutics. The current report has analyzed the dose selection approaches for 116 new molecular entities (NMEs) approved for oncology indications by the US FDA from 2010 to August 2021, with the goal to extract learnings about the ways to select the optimal dose. The analysis showed that: (1) the initial label dose was lower than the maximum tolerated dose (MTD) or maximum studied dose (MSD) in Phase 1 for the majority of approved NMEs, and that the MTD approach is no longer the mainstay for dose selection; (2) there was no dose ranging or optimization beyond Phase 1 dose escalation for ~ 80% of the NMEs; (3) integrated dose/exposure–response analyses were commonly used to justify the dose selection; (4) lack of dose optimization led to dose-related PMRs/PMCs in 14% of cases, but 82% of these did not result in change of the initial label dose; and (5) depending on properties of the NME and specific benefit/risk considerations for the target patient population, there could be different dose selection paradigms leading to identification of the appropriate clinical dose. The analysis supports the need to incorporate more robust dose optimization during oncology clinical development, through comparative assessment of benefit/risk of multiple dose levels, over a wide exposure range using therapeutically relevant endpoints and adequate sample size. On the other hand, in certain cases, data from FIP dose escalation may be adequate to support the dose selection.





Similar content being viewed by others
References
Sacks LV, Shamsuddin HH, Yasinskaya YI, Bouri K, Lanthier ML, Sherman RE (2014) Scientific and regulatory reasons for delay and denial of FDA approval of initial applications for new drugs, 2000–2012. JAMA J Am Med Assoc 311(4):378–384. https://doi.org/10.1001/jama.2013.282542
Minasian L, Rosen O, Auclair D, Rahman A, Pazdur R, Schilsky RL (2014) Optimizing dosing of oncology drugs. Clin Pharmacol Ther 96(5):572–579. https://doi.org/10.1038/clpt.2014.153
Janne PA, Kim G, Shaw AT, Sridhara R, Pazdur R, McKee AE (2016) Dose finding of small-molecule oncology drugs: optimization throughout the development life cycle. Clin Cancer Res 22(11):2613–2617. https://doi.org/10.1158/1078-0432.CCR-15-2643
Mathijssen RH, Sparreboom A, Verweij J (2014) Determining the optimal dose in the development of anticancer agents. Nat Rev Clin Oncol 11(5):272–281. https://doi.org/10.1038/nrclinonc.2014.40
Corbaux P, El-Madani M, Tod M, Peron J, Maillet D, Lopez J, Freyer G, You B (2019) Clinical efficacy of the optimal biological dose in early-phase trials of anti-cancer targeted therapies. Eur J Cancer 120:40–46. https://doi.org/10.1016/j.ejca.2019.08.002
Shah M, Rahman A, Theoret MR, Pazdur R (2021) The drug-dosing conundrum in oncology - when less is more. New Engl J Med 385(16):1445–1447. https://doi.org/10.1056/NEJMp2109826
Sachs JR, Mayawala K, Gadamsetty S, Kang SP, de Alwis DP (2016) Optimal dosing for targeted therapies in oncology: drug development cases leading by example. Clin Cancer Res 22(6):1318–1324. https://doi.org/10.1158/1078-0432.Ccr-15-1295
Lu D, Lu T, Stroh M, Graham RA, Agarwal P, Musib L, Li CC, Lum BL, Joshi A (2016) A survey of new oncology drug approvals in the USA from 2010 to 2015: a focus on optimal dose and related postmarketing activities. Cancer Chemother Pharmacol 77(3):459–476. https://doi.org/10.1007/s00280-015-2931-4
Friends of Cancer Research White Paper (2021) Optimizing dosing in oncology drug development. Friends of cancer research annual meeting 2021.
Project Optimus (2022) Reforming the dose optimization and dose selection paradigm in oncology. https://www.fda.gov/about-fda/oncology-center-excellence/project-optimus. Accessed 26 Apr 2022
Goldberg P (2021) RIP MTD: FDA to require sponsors to determine optimal dosage before initiating pivotal trials in cancer. Cancer Lett 47(23):13–14
Ratain MJSG, Tannock IF, Lichter AS (2021) Optimize the dose: an optimal step forward for FDA. Cancer Lett 47(23):15–16
(2022) Drugs@FDA: FDA-Approved Drugs https://www.accessdata.fda.gov/scripts/cder/daf/. Accessed 26 Apr 2022
(2022) Postmarket Requirements and Commitments. https://www.accessdata.fda.gov/scripts/cder/pmc/index.cfm. Accessed 26 Apr 2022
FDA (2012) ICLUSIG® (ponatinib) Approval letter(s). https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2012/203469Orig1s000ltr.pdf. Accessed 26 Apr 2022
Cortes J, Apperley J, Lomaia E, Moiraghi B, Undurraga Sutton M, Pavlovsky C, Chuah C, Sacha T, Lipton JH, Schiffer CA, McCloskey J, Hochhaus A, Rousselot P, Rosti G, de Lavallade H, Turkina A, Rojas C, Arthur CK, Maness L, Talpaz M, Mauro M, Hall T, Lu V, Srivastava S, Deininger M (2021) Ponatinib dose–ranging study in chronic-phase chronic myeloid leukemia: a randomized, open-label phase 2 clinical trial. Blood 138(21):2042–2050. https://doi.org/10.1182/blood.2021012082
ICLUSIG (2020) (ponatinib) [package insert]. ARIAD Pharmaceuticals Inc., Cambridge
FDA (2014) ZYKADIA® (ceritinib) Approval letter(s). https://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/205755Orig1s000Approv.pdf. Accessed 26 Apr 2022
Cho BC, Kim DW, Bearz A, Laurie SA, McKeage M, Borra G, Park K, Kim SW, Ghosn M, Ardizzoni A, Maiello E, Greystoke A, Yu R, Osborne K, Gu W, Scott JW, Passos VQ, Lau YY, Wrona A (2017) ASCEND-8: A randomized phase 1 study of ceritinib, 450 mg or 600 mg, taken with a low-fat meal versus 750 mg in fasted state in patients with anaplastic lymphoma kinase (ALK)-rearranged metastatic non-small cell lung cancer (NSCLC). J Thorac Oncol 12(9):1357–1367. https://doi.org/10.1016/j.jtho.2017.07.005
FDA (2014) ZYKADIA® (ceritinib) [package insert]. Novartis Pharmaceutical Corporation, East Hanover
ODOMZO® (2015) (sonidegib) [package insert]. Novartis Pharmaceutical Corporation, East Hanover
FDA (2015) ODOMZO® (sonidegib) clinical pharmacology and biopharmaceutics review. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/205266Orig1s000ClinPharmR.pdf. Accessed 26 Apr 2022
FDA (2012) XTANDI® (enzaltutamide) clinical pharmacology and biopharmaceutics review. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/203415Orig1s000ClinPharmR.pdf. Accessed 26 Apr 2022
IBRANCE® (2015) (palbociclib) [package insert]. Pfizer Inc., New York
Flaherty KT, Lorusso PM, Demichele A, Abramson VG, Courtney R, Randolph SS, Shaik MN, Wilner KD, O’Dwyer PJ, Schwartz GK (2012) Phase I, dose-escalation trial of the oral cyclin-dependent kinase 4/6 inhibitor PD 0332991, administered using a 21-day schedule in patients with advanced cancer. Clin Cancer Res 18(2):568–576. https://doi.org/10.1158/1078-0432.CCR-11-0509
FDA (2015) IBRANCE® (palbociclib) clinical pharmacology and biopharmaceutics review. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/207103Orig1s000ClinPharmR.pdf. Accessed 26 Apr 2022
Pallmann P, Bedding AW, Choodari-Oskooei B, Dimairo M, Flight L, Hampson LV, Holmes J, Mander AP, Odondi L, Sydes MR, Villar SS, Wason JMS, Weir CJ, Wheeler GM, Yap C, Jaki T (2018) Adaptive designs in clinical trials: why use them, and how to run and report them. BMC Med 16(1):29. https://doi.org/10.1186/s12916-018-1017-7
INLYTA® (2012) (axitinib) [package insert]. Pfizer Inc., New York.
JAKAFI® (2011) (ruxolitinib) [package insert]. Incyte Corp., Wilmington
Bosulif® (2012) (bosutinib) [package insert]. Pfizer Inc., New York
Acknowledgements
The authors would like to acknowledge Drs. Gianluca Nucci, Kourosh Parivar, Laurie Strawn, Sriram Krishnaswami, and Yazdi Pithavala for their valuable input and discussions related to the manuscript.
Funding
Funding was provided by Pfizer.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
RKM, CG, SD, and DY are employees of Pfizer and hold Pfizer stock or stock options.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mittapalli, R.K., Guo, C., Drescher, S.K. et al. Oncology dose optimization paradigms: knowledge gained and extrapolated from approved oncology therapeutics. Cancer Chemother Pharmacol 90, 207–216 (2022). https://doi.org/10.1007/s00280-022-04444-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-022-04444-0