Abstract
Purpose
Oraxol is an oral formulation of paclitaxel administered with a novel, minimally absorbed P-glycoprotein inhibitor encequidar (HM30181A). This phase Ib study was conducted to determine the maximum-tolerated dose (MTD) of Oraxol administered at a fixed dose for up to 5 consecutive days in patients with advanced malignancies.
Methods
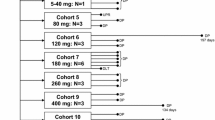
Part 1 of this study utilized a 3 + 3 dose-escalation design to determine the MTD of oral paclitaxel 270 mg plus oral encequidar 15 mg administered daily. Dose escalation was achieved by increasing the number of consecutive dosing days per week (from 2 to 5 days per week). Dosing occurred for 3 consecutive weeks out of a 4-week cycle. Part 2 treated additional patients at the MTD to determine tolerability and recommended phase II dose (RP2D). Adverse events, tumor responses, and pharmacokinetic profiles were assessed.
Results
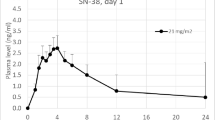
A total of 34 patients (n = 24 in Part 1, n = 10 in Part 2) received treatment. The MTD of Oraxol was determined to be 270 mg daily × 5 days per week per protocol definition and this was declared the RP2D. The most common treatment-related adverse events were fatigue, neutropenia, and nausea/vomiting. Hypersensitivity-type reactions were not observed. Of the 28 patients evaluable for response, 2 (7.1%) achieved partial response and 18 (64.3%) achieved stable disease. Pharmacokinetic analysis showed rapid absorption of paclitaxel when administered orally following encequidar. Paclitaxel daily exposure was comparable following 2–5 days dose levels.
Conclusion
The oral administration of encequidar with paclitaxel was safe, achieved clinically relevant paclitaxel levels, and showed evidence of anti-tumor activity.



Similar content being viewed by others
Data availability
The data that support the findings of this study are available on request from the corresponding author upon reasonable request.
References
Singla AK, Garg A, Aggarwal D (2002) Paclitaxel and its formulations. Int J Pharm 235:179–192. https://doi.org/10.1016/S0378-5173(01)00986-3
Gelderblom H, Verweij J, Nooter K, Sparreboom A (2001) Cremophor EL: the drawbacks and advantages of vehicle selection for drug formulation. Eur J Cancer 37:1590–1598. https://doi.org/10.1016/S0959-8049(01)00171-X
Sparreboom A, van Asperen J, Mayer U et al (1997) Limited oral bioavailability and active epithelial excretion of paclitaxel (Taxol) caused by P-glycoprotein in the intestine. Proc Natl Acad Sci U S A 94:2031–2035. https://doi.org/10.1073/pnas.94.5.2031
Bardelmeijer HA, Beijnen JH, Brouwer KR et al (2000) Increased oral bioavailability of paclitaxel by GF120918 in mice through selective modulation of P-glycoprotein. Clin Cancer Res 6:4416LP-4421LP
Paek IB, Kim SY, Kim MS et al (2007) Characterization of human liver cytochrome P-450 enzymes Involved in the O-demethylation of a new P-glycoprotein Inhibitor HM-30181. J Toxicol Environ Heal Part A 70:1356–1364. https://doi.org/10.1080/15287390701434307
Kwak J-O, Lee SH, Lee GS et al (2010) Selective inhibition of MDR1 (ABCB1) by HM30181 increases oral bioavailability and therapeutic efficacy of paclitaxel. Eur J Pharmacol 627:92–98. https://doi.org/10.1016/j.ejphar.2009.11.008
Lee HJ, Heo D-S, Cho J-Y et al (2014) A phase I study of oral paclitaxel with a novel P-Glycoprotein inhibitor, HM30181A, in patients with advanced solid cancer. Cancer Res Treat 46:234–242. https://doi.org/10.4143/crt.2014.46.3.234
Lee K-W, Lee KH, Zang DY et al (2015) Phase I/II study of weekly oraxol for the second-line treatment of patients with metastatic or recurrent gastric cancer. Oncologist 20:896–897. https://doi.org/10.1634/theoncologist.2015-0202
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumors: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247. https://doi.org/10.1016/j.ejca.2008.10.026
Malingré MM, Meerum Terwogt JM, Beijnen JH et al (2000) Phase I and pharmacokinetic study of oral paclitaxel. J Clin Oncol 18:2468–2475. https://doi.org/10.1200/JCO.2000.18.12.2468
Malingré MM, Beijnen JH, Rosing H et al (2001) A phase I and pharmacokinetic study of bi-daily dosing of oral paclitaxel in combination with cyclosporin A. Cancer Chemother Pharmacol 47:347–354. https://doi.org/10.1007/s002800000226
Kruijtzer CMF, Schellens JHM, Mezger J et al (2002) Phase II and pharmacologic study of weekly oral paclitaxel plus cyclosporine in patients with advanced non-small-cell lung cancer. J Clin Oncol 20:4508–4516. https://doi.org/10.1200/JCO.2002.04.058
Kruijtzer CMF, Boot H, Beijnen JH et al (2003) Weekly oral paclitaxel as first-line treatment in patients with advanced gastric cancer. Ann Oncol 14:197–204. https://doi.org/10.1093/ANNONC/MDG078
Veltkamp SA, Thijssen B, Garrigue JS et al (2006) (2006) A novel self-microemulsifying formulation of paclitaxel for oral administration to patients with advanced cancer. Br J Cancer 956(95):729–734. https://doi.org/10.1038/sj.bjc.6603312
Veltkamp SA, Alderden-Los C, Sharma A et al (2007) A pharmacokinetic and safety study of a novel polymeric paclitaxel formulation for oral application. Cancer Chemother Pharmacol 59:43–50. https://doi.org/10.1007/S00280-006-0245-2/TABLES/4
Hong JW, Lee I-H, Kwak YH et al (2007) Efficacy and tissue distribution of DHP107, an oral paclitaxel formulation. Mol Cancer Ther 6:3239–3247. https://doi.org/10.1158/1535-7163.MCT-07-0261
Hong YS, Kim KP, Lim HS et al (2012) (2012) A phase I study of DHP107, a mucoadhesive lipid form of oral paclitaxel, in patients with advanced solid tumors: crossover comparisons with intravenous paclitaxel. Investig New Drugs 313(31):616–622. https://doi.org/10.1007/S10637-012-9841-7
Kang YK, Ryu MH, Park SH et al (2018) Efficacy and safety findings from DREAM: a phase III study of DHP107 (oral paclitaxel) versus i.v. paclitaxel in patients with advanced gastric cancer after failure of first-line chemotherapy. Ann Oncol 29:1220–1226. https://doi.org/10.1093/ANNONC/MDY055
Ryu M, Ryoo B, Kim TW et al (2017) A phase I/IIa study of DHP107, a novel oral paclitaxel formulation, in patients with advanced solid tumors or gastric cancer. Oncologist 22:129-e8. https://doi.org/10.1634/theoncologist.2016-0273
Yang Y, Li P, Zhang Z et al (2020) Prediction of cyclosporin-mediated drug interaction using physiologically based pharmacokinetic model characterizing interplay of drug transporters and enzymes. Int J Mol Sci 21:7023. https://doi.org/10.3390/ijms21197023
Graham RM (1994) Cyclosporine: mechanisms of action and toxicity. Cleve Clin J Med 61:308LP-313LP
Pfizer. Paclitaxel [Package insert]. US food and drug administration website. www.accessdata.fda.gov/drugsatfda_docs/label/2011/020262s049lbl.pdf.
Ravi V, Wagner M, Chen TW-W et al (2020) A phase II study of oraxol in the treatment of unresectable cutaneous angiosarcoma. J Clin Oncol 38:11517. https://doi.org/10.1200/JCO.2020.38.15_suppl.11517
Celgene Corporation. Abraxane [Package insert]. US food and drug administration website. www.accessdata.fda.gov/drugsatfda_docs/label/2020/021660s047lbl.pdf
Jackson CGCA, Hung T, Segelov E et al (2021) Oral paclitaxel with encequidar compared to intravenous paclitaxel in patients with advanced cancer: a randomised crossover pharmacokinetic study. Br J Clin Pharmacol 87:4670–4680. https://doi.org/10.1111/bcp.14886
Britten CD, Baker SD, Denis LJ et al (2000) Oral paclitaxel and concurrent cyclosporin A: targeting clinically relevant systemic exposure to paclitaxel1. Clin Cancer Res 6:3459–3468
Helgason HH, Kruijtzer CMF, Huitema ADR et al (2006) Phase II and pharmacological study of oral paclitaxel (Paxoral) plus ciclosporin in anthracycline-pretreated metastatic breast cancer. Br J Cancer 95:794–800. https://doi.org/10.1038/sj.bjc.6603332
Chu Z, Chen J-S, Liau C-T et al (2008) Oral bioavailability of a novel paclitaxel formulation (Genetaxyl) administered with cyclosporin A in cancer patients. Anticancer Drugs 19:275–281. https://doi.org/10.1097/cad.0b013e3282f3fd2e
Acknowledgements
We would like to thank the patients, their families, and all the investigators who participated in this clinical trial.
Funding
Athenex, Inc provided the funding and drug for the clinical trial.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
NSA is a paid consultant for Mirati and QED; she receives institutional funding from Agios, Inc., Array, Atlas, Bayer HealthCare, BMS, Celgene, Debio, Eli Lilly and Company, EMD Serono, Incyte Corporation, Intensity, Merck & Co., Inc. and Taiho Pharmaceuticals Co., Ltd.; she participates on advisory boards for Incyte, QED, and Glaxo Smith Kline. JRD receives institutional research funding from Adlai Norte, Takeda, Gilead, Merck, AstraZeneca, Astellas, Abbvie, BMS, OnKure, Deciphera, Bayer, Hutchison, and Genentech; she is a consultant for Gilead and OnKure; she owns stock options in OnKure Therapeutics. MO receives research funding from Eli Lilly and Pfizer; he is a consultant for AstraZeneca and Novartis. AJ receives institutional research funding from Pfizer, Merck, SQZ Biotech, Moderna, Iovance, Khar, DebioPharm, Cantargia, and Sanofi; he owns stock options in Champions Oncology and Suvica. JZ, DK, WKC, DC, and RW are employees of Athenex, Inc. DC owns stock in Athenex, Inc. and Merck & Co., Inc. No conflicts of interests were disclosed by the other authors.
Ethical approval
This study was approved by the institutional review boards at each institution and was performed in accordance with the principles of the Declaration of Helsinki.
Consent to participate
Written informed consent to participate in the study was obtained from all participants included in the study.
Consent to publish
Written informed consent for publication of clinical data was obtained from all participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ma, W.W., Li, J.J., Azad, N.S. et al. A phase Ib study of Oraxol (oral paclitaxel and encequidar) in patients with advanced malignancies. Cancer Chemother Pharmacol 90, 7–17 (2022). https://doi.org/10.1007/s00280-022-04443-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-022-04443-1