Abstract
Purpose
Genetic variation in the activation of the prodrug cyclophosphamide (CP) by cytochrome P450 (CYP) enzymes has been shown to influence outcomes. However, CYP are also subject to phenoconversion due to either the effects of comedications or cancer associated down-regulation of expression. The aim of this study was to assess the relationship between CP bioactivation with CYP2B6 and CYP2C19 genotype, as well as CYP2C19 phenotype, in breast cancer patients.
Methods
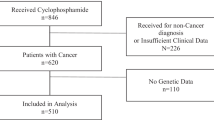
CP and the active metabolite levels were assessed in breast cancer patients (n = 34) at cycle 1 and cycle 3 of treatment. Patients were genotyped for a series of SNP known to affect CYP2B6 and CYP2C19 function. The activity of CYP2C19 was also assessed using a probe drug.
Results
We found a significant linear gene-dose relationship with CYP2B6 coding SNP and formation of 4-hydroxycyclophosphamide. A possible association with CYP2C19 null genotype at cycle 1 was obscured at cycle 3 due to the substantial intra-individual change in CP bioactivation on subsequent dosing.
Conclusion
Comedications may be the cause for this inter-occasion variation in bioactivation of cyclophosphamide and the ensuing phenoconversion may account for the conflicting reports in the literature about the relationship between CYP2C19 genotype and CP bioactivation pharmacokinetics. Trial registration ANZCTR363222 (6/11/2012, retrospectively registered).




Similar content being viewed by others
Data availability
Data are available on request to the corresponding author.
References
Helsby NA, Yong M, van Kan M et al (2019) The importance of both CYP2C19 and CYP2B6 germline variations in cyclophosphamide pharmacokinetics and clinical outcomes. Br J Clin Pharmacol 85:1925–1934. https://doi.org/10.1111/bcp.14031
Helsby NA, Hui C-Y, Goldthorpe MA et al (2010) The combined impact of CYP2C19 and CYP2B6 pharmacogenetics on cyclophosphamide bioactivation. Br J Clin Pharmacol 70:844–853. https://doi.org/10.1111/j.1365-2125.2010.03789.x
Kishino Y, Hasegawa T, Kato A et al (2019) Effect of inter-individual variability in human liver cytochrome P450 isozymes on cyclophosphamide-induced micronucleus formation. Mutat Res Toxicol Environ Mutagen 838:37–45. https://doi.org/10.1016/j.mrgentox.2018.11.016
Helsby NA, Burns KE (2012) Molecular mechanisms of genetic variation and transcriptional regulation of CYP2C19. Front Genet. https://doi.org/10.3389/fgene.2012.00206
Zanger UM, Klein K (2013) Pharmacogenetics of cytochrome P450 2B6 (CYP2B6): advances on polymorphisms, mechanisms, and clinical relevance. Front Genet. https://doi.org/10.3389/fgene.2013.00024
Klomp SD, Manson ML, Guchelaar H-J, Swen JJ (2020) Phenoconversion of cytochrome P450 metabolism: a systematic review. J Clin Med 9:2890. https://doi.org/10.3390/jcm9092890
Helsby NA, Lo W-Y, Sharples K et al (2008) CYP2C19 pharmacogenetics in advanced cancer: compromised function independent of genotype. Br J Cancer 99:1251–1255. https://doi.org/10.1038/sj.bjc.6604699
Williams ML, Bhargava P, Cherrouk I et al (2000) A discordance of the cytochrome P450 2C19 genotype and phenotype in patients with advanced cancer. Br J Clin Pharmacol 49:485–488. https://doi.org/10.1046/j.1365-2125.2000.00189.x
Burns KE, Goldthorpe MA, Porteus F et al (2014) CYP2C19 genotype–phenotype discordance in patients with multiple myeloma leads to an acquired loss of drug-metabolising activity. Cancer Chemother Pharmacol 73:651–655. https://doi.org/10.1007/s00280-014-2409-9
Burns KE, Lo W-Y, Findlay MP et al (2016) High CYP2C19 phenotypic variability in gastrointestinal cancer patients. Cancer Chemother Pharmacol 77:195–204. https://doi.org/10.1007/s00280-015-2923-4
Lang T, Klein K, Fischer J et al (2001) Extensive genetic polymorphism in the human CYP2B6 gene with impact on expression and function in human liver. Pharmacogenetics 11:399–415. https://doi.org/10.1097/00008571-200107000-00004
Xie H, Griskevicius L, Ståhle L et al (2006) Pharmacogenetics of cyclophosphamide in patients with hematological malignancies. Eur J Pharm Sci 27:54–61. https://doi.org/10.1016/j.ejps.2005.08.008
Veal GJ, Cole M, Chinnaswamy G et al (2016) Cyclophosphamide pharmacokinetics and pharmacogenetics in children with B-cell non-Hodgkin’s lymphoma. Eur J Cancer 55:56–64. https://doi.org/10.1016/j.ejca.2015.12.007
Afsar NA, Ufer M, Haenisch S et al (2012) Relationship of drug metabolizing enzyme genotype to plasma levels as well as myelotoxicity of cyclophosphamide in breast cancer patients. Eur J Clin Pharmacol 68:389–395. https://doi.org/10.1007/s00228-011-1134-0
Shu W, Chen L, Hu X et al (2017) Cytochrome P450 genetic variations can predict mRNA expression, cyclophosphamide 4-hydroxylation, and treatment outcomes in Chinese patients with non-Hodgkin’s lymphoma. J Clin Pharmacol 57:886–898. https://doi.org/10.1002/jcph.878
Jakobsen Falk I, Khan MS, Thunell L et al (2012) Association of CYP2B6 genotype with survival and progression free survival in cyclophosphamide treated multiple myeloma. J Cancer Ther 3:20–27
Johnson GG, Lin K, Cox TF et al (2013) CYP2B6*6 is an independent determinant of inferior response to fludarabine plus cyclophosphamide in chronic lymphocytic leukemia. Blood 122:4253–4258. https://doi.org/10.1182/blood-2013-07-516666
Melanson SEF, Stevenson K, Kim H et al (2010) Allelic variations in CYP2B6 and CYP2C19 and survival of patients receiving cyclophosphamide prior to myeloablative hematopoietic stem cell transplantation. Am J Hematol 85:967–971. https://doi.org/10.1002/ajh.21889
Haroun F, Al-Shaar L, Habib RH et al (2015) Effects of CYP2B6 genetic polymorphisms in patients receiving cyclophosphamide combination chemotherapy for breast cancer. Cancer Chemother Pharmacol 75:207–214. https://doi.org/10.1007/s00280-014-2632-4
Bray J, Sludden J, Griffin MJ et al (2010) Influence of pharmacogenetics on response and toxicity in breast cancer patients treated with doxorubicin and cyclophosphamide. Br J Cancer 102:1003–1009. https://doi.org/10.1038/sj.bjc.6605587
Timm R, Kaiser R, Lötsch J et al (2005) Association of cyclophosphamide pharmacokinetics to polymorphic cytochrome P450 2C19. Pharmacogenomics J 5:365–373. https://doi.org/10.1038/sj.tpj.6500330
Ekhart C, Doodeman VD, Rodenhuis S et al (2008) Influence of polymorphisms of drug metabolizing enzymes (CYP2B6, CYP2C9, CYP2C19, CYP3A4, CYP3A5, GSTA1, GSTP1, ALDH1A1 and ALDH3A1) on the pharmacokinetics of cyclophosphamide and 4-hydroxycyclophosphamide. Pharmacogenet Genomics 18:515. https://doi.org/10.1097/FPC.0b013e3282fc9766
El-Serafi I, Fares M, Abedi-Valugerdi M et al (2015) Cytochrome P450 2J2, a new key enzyme in cyclophosphamide bioactivation and a potential biomarker for hematological malignancies. Pharmacogenomics J 15:405–413. https://doi.org/10.1038/tpj.2014.82
Edwards G, Calvert RT, Crowther C et al (1980) Repeated investigations of cyclophosphamide disposition in myeloma patients receiving intermittent chemotherapy. Br J Clin Pharmacol 10:281–285. https://doi.org/10.1111/j.1365-2125.1980.tb01756.x
Batey MA, Wright JG, Azzabi A et al (2002) Population pharmacokinetics of adjuvant cyclophosphamide, methotrexate and 5-fluorouracil (CMF). Eur J Cancer 38:1081–1089. https://doi.org/10.1016/S0959-8049(02)00024-2
Moore MJ, Erlichman C, Thiessen JJ et al (1994) Variability in the pharmacokinetics of cyclophosphamide, methotrexate and 5-fluorouracil in women receiving adjuvant treatment for breast cancer. Cancer Chemother Pharmacol 33:472–476. https://doi.org/10.1007/BF00686503
Aitken AE, Morgan ET (2007) Gene-specific effects of inflammatory cytokines on cytochrome P450 2C, 2B6 and 3A4 mRNA levels in human hepatocytes. Drug Metab Dispos Biol Fate Chem 35:1687–1693. https://doi.org/10.1124/dmd.107.015511
Rivory LP, Slaviero KA, Clarke SJ (2002) Hepatic cytochrome P450 3A drug metabolism is reduced in cancer patients who have an acute-phase response. Br J Cancer 87:277–280. https://doi.org/10.1038/sj.bjc.6600448
Lenoir C, Daali Y, Rollason V et al (2020) Impact of acute inflammation on cytochromes P450 activity assessed by the Geneva Cocktail. Clin Pharmacol Ther. https://doi.org/10.1002/cpt.2146
Ming Z, Yongqiang Z, Zijin Z et al (2019) Severe and prolonged cyclophosphamide-induced hepatotoxicity in a breast cancer patient carrying a CYP2B6*7 variant. Pharmacogenomics 20:1119–1124. https://doi.org/10.2217/pgs-2019-0093
Grant MKO, Abdelgawad IY, Lewis CA, Zordoky BN (2020) Sexual dimorphism in doxorubicin-induced systemic inflammation: implications for hepatic cytochrome P450 regulation. Int J Mol Sci 21:1279. https://doi.org/10.3390/ijms21041279
de Jonge ME, Huitema ADR, Rodenhuis S, Beijnen JH (2005) Clinical pharmacokinetics of cyclophosphamide. Clin Pharmacokinet 44:1135–1164. https://doi.org/10.2165/00003088-200544110-00003
Xie H-J, Broberg U, Griskevicius L et al (2003) Alteration of pharmacokinetics of cyclophosphamide and suppression of the cytochrome p450 genes by ciprofloxacin. Bone Marrow Transplant 31:197–203. https://doi.org/10.1038/sj.bmt.1703815
Martin H, Sarsat J-P, de Waziers I et al (2003) Induction of cytochrome P450 2B6 and 3A4 expression by phenobarbital and cyclophosphamide in cultured human liver slices. Pharm Res 20:557–568. https://doi.org/10.1023/A:1023234429596
Slattery JT, Kalhorn TF, McDonald GB et al (1996) Conditioning regimen-dependent disposition of cyclophosphamide and hydroxycyclophosphamide in human marrow transplantation patients. J Clin Oncol 14:1484–1494. https://doi.org/10.1200/JCO.1996.14.5.1484
Walko CM, Combest AJ, Spasojevic I et al (2012) The effect of aprepitant and race on the pharmacokinetics of cyclophosphamide in breast cancer patients. Cancer Chemother Pharmacol 69:1189–1196. https://doi.org/10.1007/s00280-011-1815-5
Gilbert CJ, Petros WP, Vredenburgh J et al (1998) Pharmacokinetic interaction between ondansetron and cyclophosphamide during high-dose chemotherapy for breast cancer. Cancer Chemother Pharmacol 42:497–503. https://doi.org/10.1007/s002800050851
Chang TK, Yu L, Maurel P, Waxman DJ (1997) Enhanced cyclophosphamide and ifosfamide activation in primary human hepatocyte cultures: response to cytochrome P-450 inducers and autoinduction by oxazaphosphorines. Cancer Res 57:1946–1954
Lindley C, Hamilton G, McCune JS et al (2002) The effect of cyclophosphamide with and without dexamethasone on cytochrome P450 3A4 and 2B6 in human hepatocytes. Drug Metab Dispos 30:814–822. https://doi.org/10.1124/dmd.30.7.814
Moscovitz JE, Kalgutkar AS, Nulick K et al (2018) Establishing transcriptional signatures to differentiate PXR-, CAR-, and AhR-mediated regulation of drug metabolism and transport genes in cryopreserved human hepatocytes. J Pharmacol Exp Ther 365:262–271. https://doi.org/10.1124/jpet.117.247296
Yu LJ, Drewes P, Gustafsson K et al (1999) In vivo modulation of alternative pathways of P-450-catalyzed cyclophosphamide metabolism: impact on pharmacokinetics and antitumor activity. J Pharmacol Exp Ther 288:928–937
Jurima M, Inaba T, Kalow W (1985) Mephenytoin hydroxylase activity in human liver: inhibition by steroids. Drug Metab Dispos 13:746–749
Hedrich WD, Hassan HE, Wang H (2016) Insights into CYP2B6-mediated drug–drug interactions. Acta Pharm Sin B 6:413–425. https://doi.org/10.1016/j.apsb.2016.07.016
Shu W, Guan S, Yang X et al (2016) Genetic markers in CYP2C19 and CYP2B6 for prediction of cyclophosphamide’s 4-hydroxylation, efficacy and side effects in Chinese patients with systemic lupus erythematosus. Br J Clin Pharmacol 81:327–340. https://doi.org/10.1111/bcp.12800
El-Serafi I, Afsharian P, Moshfegh A et al (2015) Cytochrome P450 oxidoreductase influences CYP2B6 activity in cyclophosphamide bioactivation. PLoS ONE 10:e0141979. https://doi.org/10.1371/journal.pone.0141979
Tulsyan S, Agarwal G, Lal P, Mittal B (2014) Significant role of CYP450 genetic variants in cyclophosphamide based breast cancer treatment outcomes: a multi-analytical strategy. Clin Chim Acta 434:21–28. https://doi.org/10.1016/j.cca.2014.04.009
Kalra S, Kaur RP, Ludhiadch A et al (2018) Association of CYP2C19*2 and ALDH1A1*1/*2 variants with disease outcome in breast cancer patients: results of a global screening array. Eur J Clin Pharmacol 74:1291–1298. https://doi.org/10.1007/s00228-018-2505-6
Acknowledgements
We thank M. Goldthorpe, J-P. Yang (RIP) and C. Bonnet for technical support and research nurse support from C. Barrett and G. Wilson. We are grateful for funding support for this study from Genesis Oncology Trust and Cancer Society NZ.
Funding
We are grateful for funding support for this study from Genesis Oncology Trust and Cancer Society of New Zealand.
Author information
Authors and Affiliations
Contributions
H and P conceived and designed the study with guidance from F. P recruited patients and F provided study coordination support. H, F and P obtained funding. Y helped undertake genomic analysis under guidance of B. H, B and Y undertook data analysis. All authors contributed to manuscript writing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval and consent to participate
As noted in the main text this study received approval from the New Zealand Heath and Disability Northern X Regional Ethics committee (NTX/12/06/052). Patients were eligible for the study if they were diagnosed with carcinoma of the breast and scheduled to receive cyclophosphamide treatment. Patients had to be at least 18 years of age and able to give informed written consent. The study was performed in accordance with the Declaration of Helsinki.
Consent to publication
All authors consent to publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Helsby, N., Yong, M., Burns, K. et al. Cyclophosphamide bioactivation pharmacogenetics in breast cancer patients. Cancer Chemother Pharmacol 88, 533–542 (2021). https://doi.org/10.1007/s00280-021-04307-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-021-04307-0