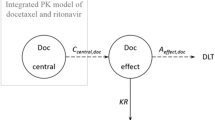
Abstract
Purpose
ModraDoc006 is a novel oral formulation of docetaxel. The clearance of intravenous docetaxel is higher in medically castrated prostate cancer patients as compared to patients with other types of solid tumours. Oral docetaxel requires co-administration ritonavir (r), which might further impact the pharmacokinetics (PK). We now compare the PK of docetaxel and ritonavir between patients with Hormone Sensitive Prostate Cancer (HSPC), metastatic Castration-Resistant Prostate Cancer (mCRPC) and other metastatic solid tumours, treated on the same dose and weekly schedule of ModraDoc006/r.
Methods
The docetaxel and ritonavir PK were compared between four patient groups from three clinical phase I trials, including eight male and eight female patients with different types of solid tumours (study 1), seven patients with HSPC (study 2) and five patients with mCRPC (study 3). All patients were treated with ModraDoc006 30 mg and ritonavir 100 mg in the morning, followed by ModraDoc006 20 mg and ritonavir 100 mg in the evening (ModraDoc006/r 30–20/100–100). For comparative purposes, the PK of six mCRPC patients that received 30–20/200–100 in study 3 were also evaluated.
Results
The maximum plasma concentration (Cmax) was significantly lower for both docetaxel and ritonavir in the prostate cancer patients as compared to the patients with other types of solid tumours treated at ModraDoc006/r 30–20/100–100. The docetaxel area under the plasma concentration versus time curve (AUC) was significantly different at this dose, with a mean AUC0-48 of 1359 ± 374 ng/mL*h (N = 8) in female patients and 894 ± 223 ng/mL*h (N = 8) in male patients with different solid tumours (study 1), 321 ± 81 (N = 7) in HSPC (study 2) and 367 ± 182 ng/mL*h (N = 5) in mCRPC (study 3). A similar pattern was observed for ritonavir. ModraDoc006/r 30–20/200–100 in six mCRPC patients led to a comparable ritonavir exposure as compared to the patients at 30–20/100–100 in study 1 and increased the docetaxel AUC0–48 to 1266 ± 473 ng/mL*h (N = 6).
Conclusion
The exposure to docetaxel and ritonavir was significantly lower in prostate cancer patients as compared to patients with other types of solid tumours, treated on ModraDoc006/r 30–20/100–100. An increase of the ritonavir dose increased the docetaxel exposure in mCRPC patients. Therefore, a different RP2D of ModraDoc006/r is pursued in castrated prostate cancer patients as compared to patients with other types of solid tumours.
Trial registration
Study 1: ClinicalTrials.gov Identifier NCT01173913, date of registration August 2, 2010. Study 2: ClinicalTrials.gov Identifier NCT03066154, date of registration February 28, 2017. Study 3: ClinicalTrials.gov Identifier NCT03136640, date of registration May 2, 2017.



Similar content being viewed by others
Availability of data and material
Additional data are available upon reasonable request to the authors.
Code availability
This is not applicable for this manuscript.
References
Sanofi-Aventis (2005) Taxotere, INN-docetaxel—European Medicines Agency. Summary of product characteristics. https://www.ema.europa.eu/en/medicines/human/EPAR/taxotere. Accessed 01 Dec 2020
Tannock IF, de Wit R, Berry WR et al (2004) Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 351:1502–1512
Vale CL, Burdett S, Rydzewska LHM et al (2016) Addition of docetaxel or bisphosphonates to standard of care in men with localised or metastatic, hormone-sensitive prostate cancer: a systematic review and meta-analyses of aggregate data. Lancet Oncol 17:243–256
Horn L, Spigel DR, Vokes EE et al (2017) Nivolumab versus docetaxel in previously treated patients with advanced non-small-cell lung cancer: two-year outcomes from two randomized, open-label, phase III trials (CheckMate 017 and CheckMate 057). J Clin Oncol 35:3924–3933
Sparano JA (2000) Taxanes for breast cancer: an evidence-based review of randomized phase II and phase III trials. Clin Breast Cancer 1:32–40
De Vries Schultink AHM, Crombag MBS, van Werkhoven E et al (2019) Neutropenia and docetaxel exposure in metastatic castration-resistant prostate cancer patients: a meta-analysis and evaluation of a clinical cohort. Cancer Med 8:1406–1415
Flores JP, Saif MW (2013) Novel oral taxane therapies: recent phase I results. Clin Investig (Lond) 3:333–341
Van Waterschoot RA, Lagas JS, Wagenaar E, van der Kruijssen CM, van Herwaarden AE, Song JY et al (2009) Abscence of both cytochrome P450 3A and P-glycoprotein dramatically increases docetaxel oral bioavailability and risk of intestinal toxicity. Cancer Res 69:8996–9002
Bardelmeijer HA, Ouwehand M, Buckle T, Huisman MT, Schellens JHM, Beijnen JH et al (2002) Low systemic exposure of oral docetaxel in mice resulting from extensive first-pass metabolism is boosted by ritonavir. Cancer Res 62:6158–6164
Oostendorp RL, Huitema A, Rosing H, Jansen RS, Ter Heine R, Keessen M et al (2009) Coadministration of ritonavir strongly enhances the apparent oral bioavailability of docetaxel in patients with solid tumors. Clin Cancer Res 15:4228–4233
De Weger VA, Stuurman FE, Koolen SLW, Moes JJ, Hendrikx JJMA, Sawicki E et al (2019) A phase I dose escalation study of once-weekly oral administration of docetaxel as ModraDoc001 capsule or ModraDoc006 tablet in combination with ritonavir. Clin Cancer Res 25:5466–5474
De Weger VA, Stuurman FE, Hendrikx J et al (2017) A dose-escalation study of bi-daily once weekly oral docetaxel either as ModraDoc001 or ModraDoc006 combined with ritonavir. Eur J Cancer 86:217–225
Vermunt MAC, Janssen JM, Vrijenhoek GL, Van der Poel HG, Thijssen B, Beijnen JH et al (2019) Addition of an oral docetaxel treatment (ModraDoc006/r) to androgen deprivation therapy (ADT) and intensity-modulated radiation therapy (IMRT) in patients with high risk N+M0 prostate cancer. Ann Oncol 30:v325–v355
Vermunt M, Robbrecht D, Devriese L, Janssen J, Keessen M, Eskens F et al (2021) ModraDoc006, an oral docetaxel formulation in combination with ritonavir (ModraDoc006/r), in metastatic castration-resistant prostate cancer patients: a phase Ib study. Cancer Rep (Hoboken). https://doi.org/10.1002/cnr2.1367
ModraDoc006/r in patients with breast cancer. Identifier NCT03890744. https://www.clinicaltrials.gov. Accessed 01 Dec 2020
Vaishampayan UN, De Wit EJ, Shore ND, Dreicer R, George DJ, Boccia RV et al (2020) A multicenter phase IIb trial to evaluate the efficacy and tolerability of ModraDoc006/r in subjects with metastatic castration-resistant prostate cancer (mCRPC), suitable for treatment with a taxane (NCT04028388). J Clin Oncol 38:268–268
Sawicki E, Beijnen JH, Schellens JHM, Nuijen B (2016) Pharmaceutical development of an oral tablet formulation containing a spray dried amorphous solid dispersion of docetaxel or paclitaxel. Int J Pharm 511:765–773
Hendrikx JJ, Hillebrand MJ, Thijssen B, Rosing H, Schinkel AH, Schellens JHM, Beijnen JH (2011) A sensitive combined assay for the quantification of paclitaxel, docetaxel and ritonavir in human plasma using liquid chromatography coupled with tandem mass spectrometry. J Chromatogr B Anal Technol Biomed Life Sci 879:2984–2990
RC Team (2009) R: a language and environment for statistical computing. R foundation for statistical computing, Vienna. https://www.r-project.org. Accessed 01 Dec 2020
Van Waterschoot RA, Lagas JS, Wagenaar E, Rosing H, Beijnen JH, Schinkel AH (2010) Individual and combined roles of CYP3A, P-glycoprotein (MDR1/ABCB1) and MRP2 (ABBC2) in the pharmacokinetics of docetaxel. Int J Cancer 127:2959–2964
Iusuf D, Hendrikx JJ, van Esch A, van de Steeg E, Wagenaar E, Rosing H et al (2015) Human OATP1B1, OATP1B3 and OATP1A2 can mediate the in vivo uptake and clearance of docetaxel. Int J Cancer 136:225–233
Hsu A, Granneman GR, Bertz RJ (1998) Ritonavir. Clinical pharmacokinetics and interactions with other anti-HIV agents. Clin Pharmacokinet 35:275–291
Sevrioukova IF, Poulos TL (2010) Structure and mechanism of the complex between cytochrome P4503A4 and ritonavir. Proc Natl Acad Sci USA 107:18422–18427
Zhou SF (2008) Drugs behave as substrates, inhibitors and inducers of human cytochrome P450 3A4. Curr Drug Metab 9:310–322
Nieuweboer AJM, de Morrée ES, de Graan AJM, Sparreboom A, de Wit R, Mathijssen RHJ (2015) Inter-patient variability in docetaxel pharmacokinetics: a review. Cancer Treat Rev 41:605–613
Cummins CL, Chi-Yuan Wu, Benet LZ (2002) Sex-related differences in the clearance of cytochrome P450 3A4 substrates may be caused by P-glycoprotein. Clin Pharmacol Ther 72:474–489
Franke RM, Carducci MA, Rudek MA, Baker SD, Sparreboom A (2010) Castration-dependent pharmacokinetics of docetaxel in patients with prostate cancer. J Clin Oncol 28:4562–4567
Hutson PR, Oettel K, Douglas J, Ritter M, Messing E et al (2008) Effect of medical castration on CYP3A4 activity using the erythromycin breath test. Cancer Chemother Pharmacol 62:373–377
Mathijssen RHJ, van Schaik RHN (2006) Genotyping and phenotyping cytochrome P450: perspectives for cancer treatment. Eur J Cancer 42:141–148
Söderberg Löfdal KC, Andersson ML, Gustafsson LL (2013) Cytochrome P450-mediated changes in oxycodone pharmacokinetics/pharmacodynamics and their clinical implications. Drugs 73:533–543
Kuip EJM, Zandvliet ML, Koolen SLW, Mathijssen RHJ, van der Rijt CCD (2017) A review of factors explaining variability in fentanyl pharmacokinetics; focus on implications for cancer patients. Br J Clin Pharmacol 83:294–313
Franco-Salinas G, de la Rosette JJMCH, Michel MC (2010) Pharmacokinetics and pharmacodynamics of tamsulosin in its modified-release and oral controlled absorption system formulations. Clin Pharmacokinet 49:177–188
Westdorp H, Kuip EJM, van Oort IM, Kramers C, Gerritsen WR, Vissers KCP (2018) Difficulties in pain management using oxycodone and fentanyl in enzalutamide-treated patients with advanced prostate cancer. J Pain Symptom Manag 55:e6-8
Streetman DS, Bertino JS, Nafziger AN (2000) Phenotyping of drug-metabolizing enzymes in adults: a review of in-vivo cytochrome P450 phenotyping probes. Pharmacogenetics 10:187–216
Hohmann N, Haefeli WE, Mikus G (2016) CYP3A activity: towards dose adaptation to the individual. Expert Opin Drug Metab Toxicol 12:479–497
Fuhr U, Jetter A, Kirchheiner J (2007) Appropriate phenotyping procedures for drug metabolizing enzymes and transporters in humans and their simultaneous use in the “cocktail” approach. Clin Pharmacol Ther 81:270–283
Sissung TM, Ley AM, Strope JD, McCrea EM, Beedie S, Peer CJ et al (2017) Differential expression of OATP1B3 mediates unconjugated testosterone influx. Mol Cancer Res 15:1096–1105
De Morrée ES, Böttcher R, van Soest RJ, Aghai A, de Ridder CM, Gibson AA et al (2016) Loss of SLCO1B3 drives taxane resistance in prostate cancer. Br J Cancer 115:674–681
Mout L, Moll JM, Chen M, de Morrée ES, de Ridder CMA, Gibson A et al (2020) Androgen receptor signaling impairs docetaxel efficacy in castration-resistant prostate cancer. Br J Cancer 123:1715–1719
De Bruyn T, Stieger B, Augustijns PF, Annaert PP (2016) Clearance prediction of HIV protease inhibitors in man: role of hepatic uptake. J Pharm Sci 105:854–863
Shou M, Martinet M, Korzekwa KR, Krausz KW, Gonzalez FJ, Gelboin HV (1998) Role of human cytochrome P450 3A4 and 3A5 in the metabolism of taxotere and its derivatives: enzyme specificity, interindividual distribution and metabolic contribution in human liver. Pharmacogenetics 8:394–401
Moilanen AM, Hakkola J, Vaarala MH, Kauppila S, Hirvikoski P, Vuoristo J et al (2007) Characterization of androgen-regulated expression of CYP3A5 in human prostate. Carcinogenesis 28:916–921
Funding
Study 1 and 2 are investigator initiated trials funded by the Netherlands Cancer Institute. Jos H Beijnen has received a grand for translational research (ZonMw code 40-41200-98-004). Study 3 was funded by Modra Pharmaceuticals BV, a spin-off company from the Netherlands Cancer Institute. For this manuscript, no additional funding was obtained.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Jos H Beijnen is a part time employee and shareholder of Modra Pharmaceuticals BV and is a patent holder on oral taxane formulations. Eric van der Putten is a part-time employee and director of Modra Pharmaceuticals BV and is a partner at Aglaia Oncology Funds, which has investments in Modra Pharmaceuticals BV. The other authors declare that they have no conflict of interest.
Ethics approval
The three studies were approved by the Medical Ethical Committee of the Netherlands Cancer Institute. The studies were performed in line with the principles of the Declaration of Helsinki.
Consent to participate
All patients provided written informed consent before start of any study procedures according to Good Clinical Practice.
Consent for publication
Since all data are anonymized before publication, no additional consents are needed for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vermunt, M.A.C., van der Heijden, L.T., Hendrikx, J.J.M.A. et al. Pharmacokinetics of docetaxel and ritonavir after oral administration of ModraDoc006/r in patients with prostate cancer versus patients with other advanced solid tumours. Cancer Chemother Pharmacol 87, 855–869 (2021). https://doi.org/10.1007/s00280-021-04259-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-021-04259-5