Abstract
Purpose
Temozolomide is the most effective chemotherapy for malignant glioma. Hypersensitivity requiring interruption of therapy may significantly impact patient survival. We have successfully employed temozolomide desensitization followed by metronomic dosing of temozolomide. Our purpose was to report patient characteristics and outcomes in patients with glioma (Grade 2–4) and temozolomide hypersensitivity managed by desensitization and metronomic dosing.
Methods
We performed an observational study of 15 patients at Mayo Clinic (Rochester) with a diagnosis of glioma who underwent temozolomide desensitization with subsequent metronomic dosing from May 2012 to January 2017. We calculated overall and progression-free survival using the Kaplan–Meier method, and log-rank analyses to assess for differences in survival by WHO Grade or treatment initiation.
Results
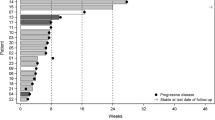
Median age at time of desensitization was 49.3 years (26.8–64.7 years). Median follow-up after desensitization was 35.5 months. One patient (6.7%) was unable to resume temozolomide due to recurrent allergy. The median time from first desensitization to discontinuation of metronomic temozolomide was 4.2 months (0–15.2 months). Median OS and PFS for the whole sample were 181.7 months and 44.9 months. For Grade 4, OS was 100% at 1 year, 40% at 3 years, 20% at 5 years; and PFS was 60% at 1 year, 40% at 3 years, and 20% at 5 years.
Conclusion
Our results suggest that rapid-desensitization followed by metronomic temozolomide should be considered in patients with glioma who experience hypersensitivity. This strategy provides comparable outcomes to therapy with standard protocols, with the majority of patients able to tolerate temozolomide after desensitization with favorable disease control.

Similar content being viewed by others

References
FDA (2015) TEMODAR: highlights of prescribing information. https://bit.ly/35uRsHk(fda.gov)
Villano JL, Seery TE, Bressler LR (2009) Temozolomide in malignant gliomas: current use and future targets. Cancer Chemother Pharmacol 64(4):647–655. https://doi.org/10.1007/s00280-009-1050-5
Newlands ES, Stevens MF, Wedge SR, Wheelhouse RT, Brock C (1997) Temozolomide: a review of its discovery, chemical properties, pre-clinical development and clinical trials. Cancer Treat Rev 23(1):35–61. https://doi.org/10.1016/s0305-7372(97)90019-0
Dixit S, Baker L, Walmsley V, Hingorani M (2012) Temozolomide-related idiosyncratic and other uncommon toxicities: a systematic review. Anticancer Drugs 23(10):1099–1106. https://doi.org/10.1097/CAD.0b013e328356f5b0
Koschel D, Handzhiev S, Leucht V, Holotiuk O, Fisseler-Eckhoff A, Höffken G (2009) Hypersensitivity pneumonitis associated with the use of temozolomide. Eur Respir J 33(4):931–934
Sarma N (2009) Stevens-Johnson Syndrome and toxic epidermal necrolysis overlap due to oral temozolomide and cranial radiotherapy. Am J Clin Dermatol 10(4):264–267. https://doi.org/10.2165/00128071-200910040-00007
Deluche E, Leobon S, Touraine F, Clavère P (2014) Two cases of cutaneous drug eruption associated with temozolomide therapy for glioblastoma. Curr Oncol (Toronto, Ont) 21(6):e779–781. https://doi.org/10.3747/co.21.2133
Virmani P, Chung E, Thomas AA, Mellinghoff IK, Marchetti MA (2015) Cutaneous adverse drug reaction associated with oral temozolomide presenting as dermal and subcutaneous plaques and nodules. JAAD Case Rep 1(5):286–288. https://doi.org/10.1016/j.jdcr.2015.06.012
Pothiawala S, Hsu MY, Yang C, Kesari S, Ibrahimi OA (2010) Urticarial hypersensitivity reaction caused by temozolomide. J Drugs Dermatol JDD 9(9):1142–1144
Alonso-Llamazares A, Vega-Castro A, Beitia-Mazuecos JM, Mateo-Borrega B, Cardenas-Contreras R (2012) Rapid desensitization with temozolomide in patients with delayed maculopapular rash. J Investig Allergol Clin Immunol 22(6):448–449
Clayton E, Madamba J, Kong XT, Braskett M (2014) Successful desensitization protocol for delayed cutaneous eruption to temozolomide. J Allergy Clin Immunol In Pract 2(5):626–628. https://doi.org/10.1016/j.jaip.2014.03.016
Mhanna H, Jiménez Blanco A, Valdez Tejeda M, Jukic Beteta K, Sánchez González M, Fernandez Rodriguez C, Vives Conesa R (2011) Desensitization to temozolomide. J Allergy Clin Immunol 127(2):197. https://doi.org/10.1016/j.jaci.2010.12.783
Mónica R-G, De las Heras Gozalo M, de la Barrera EHG, Dominguez JS (2011) Successful desensitization with temozolomide. Ann Allergy Asthma Immunol 106(6):541–542. https://doi.org/10.1016/j.anai.2011.02.009
Divekar R, Butterfield J, Maddox D (2016) Successful rapid desensitization to temozolomide: a case series. J Allergy Clin Immunol In Pract 4(3):545–546. https://doi.org/10.1016/j.jaip.2015.12.007
Kong D-S, Lee J-I, Kim WS, Son MJ, Lim DH, Kim ST, Park K, Kim JH, Eoh W, Nam D-H (2006) A pilot study of metronomic temozolomide treatment in patients with recurrent temozolomide-refractory glioblastoma. Oncol Rep 16(5):1117–1121
Kong DS, Lee JI, Kim JH, Kim ST, Kim WS, Suh YL, Dong SM, Nam DH (2010) Phase II trial of low-dose continuous (metronomic) treatment of temozolomide for recurrent glioblastoma. Neuro Oncol 12(3):289–296. https://doi.org/10.1093/neuonc/nop030
Omuro A, Chan TA, Abrey LE, Khasraw M, Reiner AS, Kaley TJ, Deangelis LM, Lassman AB, Nolan CP, Gavrilovic IT, Hormigo A, Salvant C, Heguy A, Kaufman A, Huse JT, Panageas KS, Hottinger AF, Mellinghoff I (2013) Phase II trial of continuous low-dose temozolomide for patients with recurrent malignant glioma. Neuro Oncol 15(2):242–250. https://doi.org/10.1093/neuonc/nos295
Wong ET, Timmons J, Callahan A, O'Loughlin L, Giarusso B, Alsop DC (2016) Phase I study of low-dose metronomic temozolomide for recurrent malignant gliomas. BMC Cancer 16(1):914. https://doi.org/10.1186/s12885-016-2945-2
Woo JY, Yang SH, Lee YS, Lee SY, Kim J, Hong YK (2015) Continuous low-dose temozolomide chemotherapy and microvessel density in recurrent glioblastoma. J Korean Neurosurg Soc 58(5):426–431. https://doi.org/10.3340/jkns.2015.58.5.426
Simsek C, Esin E, Yalcin S (2019) Metronomic chemotherapy: a systematic review of the literature and clinical experience. J Oncol 2019:5483791. https://doi.org/10.1155/2019/5483791
Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, Hau P, Brandes AA, Gijtenbeek J, Marosi C, Vecht CJ, Mokhtari K, Wesseling P, Villa S, Eisenhauer E, Gorlia T, Weller M, Lacombe D, Cairncross JG, Mirimanoff RO (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 10(5):459–466. https://doi.org/10.1016/s1470-2045(09)70025-7
Bent MJVD, Erridge S, Vogelbaum MA, Nowak AK, Sanson M, Brandes AA, Wick W, Clement PM, Baurain J-F, Mason WP, Wheeler H, Weller M, Aldape KD, Wesseling P, Kros JM, Tesileanu M, Golfinopoulos V, Gorlia T, Baumert BG, French P (2019) Second interim and first molecular analysis of the EORTC randomized phase III intergroup CATNON trial on concurrent and adjuvant temozolomide in anaplastic glioma without 1p/19q codeletion. J Clin Oncol 37(15_suppl):2000–2000. https://doi.org/10.1200/JCO.2019.37.15_suppl.2000
Acknowledgements
We thank all patients that were included in this study.
Funding
No funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest pertaining to this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Neth, B.J., Ruff, M.W., Uhm, J.H. et al. Temozolomide desensitization followed by metronomic dosing in patients with hypersensitivity. Cancer Chemother Pharmacol 86, 375–382 (2020). https://doi.org/10.1007/s00280-020-04123-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-020-04123-y