Abstract
Purpose
To evaluate the effects of multiple doses of loperamide on the pharmacokinetics and safety of a single oral dose of neratinib.
Methods
This was an open-label, two-period, fixed-sequence study. Twenty healthy adult subjects received an oral dose of neratinib 240 mg daily on Days 1–4 of Period 1 followed by a 7-day washout. In Period 2, oral neratinib 240 mg was administered with loperamide 4 mg followed by two further doses of loperamide 2 mg 8 and 16 h later on Days 1–4. Pharmacokinetic sampling was performed for 72 h following each neratinib dose. Safety was monitored throughout the study.
Results
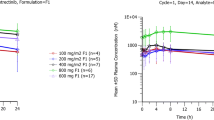
A median tmax of ~ 6 h was observed for neratinib during both periods. Apparent clearance and volume of distribution were similar for Periods 1 and 2: mean CLss/F 308.2 and 322.1 L/h; mean Vzτ/F 7995 and 10,318 L, respectively. The half-life of neratinib increased in the presence of loperamide from 18.0 to 22.2 h. Mean exposure was within the same range without and with loperamide administration: Cmax 61.2 ng/mL and 49.5 ng/mL; AUClast 1086 ng h/mL and 1153 ng h/mL, and AUCtau 779 ng h/mL and 745 ng h/mL, respectively. Treatment-emergent adverse events were mainly mild in intensity, with the most frequent events being diarrhea (45%) and constipation (35%).
Conclusions
Neratinib administered alone and concomitantly with multiple oral doses of loperamide is generally safe and well tolerated. Loperamide has minimal effects on neratinib pharmacokinetic parameters.


Similar content being viewed by others
References
Rabindran SK (2005) Antitumor activity of HER-2 inhibitors. Cancer Lett 227:9–23. https://doi.org/10.1016/j.canlet.2004.11.015
Rabindran SK, Discafani CM, Rosfjord EC et al (2004) Antitumor activity of HKI-272, an orally active, irreversible inhibitor of the HER-2 tyrosine kinase. Cancer Res 64:3958–3965. https://doi.org/10.1158/0008-5472.CAN-03-2868
Wong KK, Fracasso PM, Bukowski RM et al (2009) A phase I study with neratinib (HKI-272), an irreversible pan ErbB receptor tyrosine kinase inhibitor, in patients with solid tumors. Clin Cancer Res 15:2552–2558. https://doi.org/10.1158/1078-0432.CCR-08-1978
Burstein HJ, Sun Y, Dirix LY et al (2010) Neratinib, an irreversible ErbB receptor tyrosine kinase inhibitor, in patients with advanced ErbB2-positive breast cancer. J Clin Oncol 28:1301–1307. https://doi.org/10.1200/JCO.2009.25.8707
Martin M, Bonneterre J, Geyer CE Jr et al (2013) A phase two randomised trial of neratinib monotherapy versus lapatinib plus capecitabine combination therapy in patients with HER2 + advanced breast cancer. Eur J Cancer 49:3763–3772. https://doi.org/10.1016/j.ejca.2013.07.142
Awada A, Colomer R, Inoue K et al (2016) Neratinib plus paclitaxel vs trastuzumab plus paclitaxel in previously untreated metastatic ERBB2-positive breast cancer: the NEfERT-T randomized clinical trial. JAMA Oncol 2:1557–1564. https://doi.org/10.1001/jamaoncol.2016.0237
Awada A, Dirix L, Manso Sanchez L et al (2013) Safety and efficacy of neratinib (HKI-272) plus vinorelbine in the treatment of patients with ErbB2-positive metastatic breast cancer pretreated with anti-HER2 therapy. Ann Oncol 24:109–116. https://doi.org/10.1093/annonc/mds284
Jankowitz RC, Abraham J, Tan AR et al (2013) Safety and efficacy of neratinib in combination with weekly paclitaxel and trastuzumab in women with metastatic HER2-positive breast cancer: an NSABP Foundation Research Program phase I study. Cancer Chemother Pharmacol 72:1205–1212. https://doi.org/10.1007/s00280-013-2262-2
Saura C, Garcia-Saenz JA, Xu B et al (2014) Safety and efficacy of neratinib in combination with capecitabine in patients with metastatic human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol 32:3626–3633. https://doi.org/10.1200/JCO.2014.56.3809
Chow LW, Xu B, Gupta S et al (2013) Combination neratinib (HKI-272) and paclitaxel therapy in patients with HER2-positive metastatic breast cancer. Br J Cancer 108:1985–1993. https://doi.org/10.1038/bjc.2013.178
Chan A, Delaloge S, Holmes FA et al (2016) Neratinib after trastuzumab-based adjuvant therapy in patients with HER2-positive breast cancer (ExteNET): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 17:367–377. https://doi.org/10.1016/S1470-2045(15)00551-3
Martin M, Holmes FA, Ejlertsen B et al (2017) Neratinib after trastuzumab-based adjuvant therapy in HER2-positive breast cancer (ExteNET): 5-year analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 18:1688–1700. https://doi.org/10.1016/S1470-2045(17)30717-9
Hirsh V, Blais N, Burkes R, Verma S, Croitoru K (2014) Management of diarrhea induced by epidermal growth factor receptor tyrosine kinase inhibitors. Curr Oncol 21:329–336. https://doi.org/10.3747/co.21.2241
Ustaris F, Saura C, Di Palma J, Bryce R, Moran S, Neuman L, Ruiz R (2015) Effective management and prevention of neratinib-induced diarrhea. Am J Hematol Oncol 11:13–22
Borst P, Schinkel AH (2013) P-glycoprotein ABCB1: a major player in drug handling by mammals. J Clin Investig 123:4131–4133. https://doi.org/10.1172/jci70430
Puma Biotechnology, Inc. (2017) NERLYNX prescribing information. https://nerlynx.com/pdf/full-prescribing-information.pdf. Accessed 06 Feb 2018
Puma Biotechnology. Data on File
Niemi M, Tornio A, Pasanen MK, Fredrikson H, Neuvonen PJ, Backman JT (2006) Itraconazole, gemfibrozil and their combination markedly raise the plasma concentrations of loperamide. Eur J Clin Pharmacol 62:463–472. https://doi.org/10.1007/s00228-006-0133-z
Vandenbossche J, Huisman M, Xu Y, Sanderson-Bongiovanni D, Soons P (2010) Loperamide and P-glycoprotein inhibition: assessment of the clinical relevance. J Pharm Pharmacol 62:401–412. https://doi.org/10.1211/jpp.62.04.0001
Secombe KR, Ball IA, Shirren J, Wignall AD, Wardill HR, Van Sebille YZA, Bowen JM (2017) Budesonide reduces neratinib-induced gastrointestinal injury and diarrhoea in rats. Asia Pac J Clin Oncol 13:60–105. https://doi.org/10.1111/ajco.12798
Ibrahim E, Tripathy D, Wilkinson M, Hurvitz S, Iannotti N, Kellum A, Manalo Y, Wong S, Hansen V, Alvarez R, H., Chan A, Gore I, Kendall SD, Wade JL, Olek E, Hunt D, Fang P, Ebtahaj A, Bose R, Barcenas CH (2017) Effects of adding budesonide or colestipol to loperamide prophylaxis on neratinib-associated diarrhea in patients with HER2 + early-stage breast cancer: the CONTROL trial. Paper presented at the American Association for Cancer Research, Washington DC, USA, April 1–5, 2017 (abstr CT128)
Acknowledgements
Puma Biotechnology Inc. funded the provision of editorial support provided by Lee Miller of Miller Medical Communications.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
At the time the research was conducted, Kiana Keyvanjah, Blaire Cooke, David Martin, Dan DiPrimeo, Jane Liang, Elizabeth Olek, and Alvin Wong were employees/stock holders of the study sponsor (Puma Biotechnology Inc.). Laura Sterling and Igor Rubets have no conflicts of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Keyvanjah, K., Cooke, B., Martin, D. et al. Pharmacokinetics and safety of neratinib during co-administration with loperamide in healthy subjects. Cancer Chemother Pharmacol 84, 1125–1132 (2019). https://doi.org/10.1007/s00280-019-03951-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-019-03951-x