Abstract
Purpose
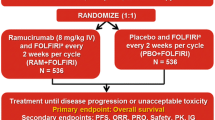
Ramucirumab plus docetaxel improved survival in REVEL, a randomized phase 3 trial for patients with Stage IV non-small cell lung cancer after standard platinum-based chemotherapy. This exploratory analysis evaluated the exposure–response relationship of ramucirumab from REVEL.
Methods
Patients received ramucirumab (10 mg/kg) or placebo plus docetaxel (75 mg/m2) every 3 weeks. Pharmacokinetic samples were collected. A population pharmacokinetic analysis predicted ramucirumab minimum concentration after first-dose administration (Cmin,1) and average concentration at steady state (Cave,ss). Predicted Cmin,1 and Cave,ss were used to evaluate the relationship between ramucirumab exposure and efficacy and safety, respectively. Exposure–efficacy was assessed by Kaplan–Meier and Cox regression analyses; exposure–safety was assessed by ordered categorical analyses.
Results
Analyses included 376 patients treated with ramucirumab plus docetaxel and 366 patients treated with placebo plus docetaxel (364 for safety population). After adjusting for corresponding prognostic factors, the association between overall survival (OS) and Cmin,1 was statistically significant (p = 0.0110), although progression-free survival (PFS) showed a marginal association (p = 0.0515). At high ramucirumab exposures (Cmin,1), greater improvements (smaller hazard ratios) were seen for OS and PFS when stratified by Cmin,1 exposure quartiles. A statistically significant correlation was observed between ramucirumab Cave,ss and grade ≥ 3 febrile neutropenia and hypertension.
Conclusions
An association was observed between ramucirumab exposure and efficacy. Higher ramucirumab exposure was associated with improved clinical outcomes and increased toxicity in this analysis. Two exposure–response prospective randomized trials are being conducted to address causation (NCT02443883 and NCT02514551), with encouraging preliminary results (Ajani et al. in Ann Oncol 28:abstr 698P, 2017).



Similar content being viewed by others
References
Spratlin JL, Mulder KE, Mackey JR (2010) Ramucirumab (IMC-1121B): a novel attack on angiogenesis. Future Oncol 6:1085–1094. https://doi.org/10.2217/fon.10.75
Spratlin JL, Cohen RB, Eadens M, Gore L, Camidge DR, Diab S, Leong S, O’Bryant C, Chow LQ, Serkova NJ, Meropol NJ, Lewis NL, Chiorean EG, Fox F, Youssoufian H, Rowinsky EK, Eckhardt SG (2010) Phase I pharmacologic and biologic study of ramucirumab (IMC-1121B), a fully human immunoglobulin G1 monoclonal antibody targeting the vascular endothelial growth factor receptor-2. J Clin Oncol 28:780–787. https://doi.org/10.1200/JCO.2009.23.7537
Garon EB, Ciuleanu TE, Arrieta O, Prabhash K, Syrigos KN, Goksel T, Park K, Gorbunova V, Kowalyszyn RD, Pikiel J, Czyzewicz G, Orlov SV, Lewanski CR, Thomas M, Bidoli P, Dakhil S, Gans S, Kim JH, Grigorescu A, Karaseva N, Reck M, Cappuzzo F, Alexandris E, Sashegyi A, Yurasov S, Perol M (2014) Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet 384:665–673. https://doi.org/10.1016/S0140-6736(14)60845-X
Larkins E, Scepura B, Blumenthal GM, Bloomquist E, Tang S, Biable M, Kluetz P, Keegan P, Pazdur R (2015) U.S. Food and Drug Administration approval summary: ramucirumab for the treatment of metastatic non-small cell lung cancer following disease progression on or after platinum-based chemotherapy. Oncologist 20:1320–1325. https://doi.org/10.1634/theoncologist.2015-0221
U.S. Department of Health and Human Services (2003) Guidance for industry, exposure–response relationships-study design, data analysis, and regulatory applications. http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm072109.pdf. Accessed 30 Aug 2015
Thurber GM, Schmidt MM, Wittrup KD (2008) Antibody tumor penetration: transport opposed by systemic and antigen-mediated clearance. Adv Drug Deliv Rev 60:1421–1434. https://doi.org/10.1016/j.addr.2008.04.012
Cosson VF, Ng VW, Lehle M, Lum BL (2014) Population pharmacokinetics and exposure–response analyses of trastuzumab in patients with advanced gastric or gastroesophageal junction cancer. Cancer Chemother Pharmacol 73:737–747. https://doi.org/10.1007/s00280-014-2400-5
Feng Y, Roy A, Masson E, Chen TT, Humphrey R, Weber JS (2013) Exposure–response relationships of the efficacy and safety of ipilimumab in patients with advanced melanoma. Clin Cancer Res 19:3977–3986. https://doi.org/10.1158/1078-0432.CCR-12-3243
Wang J, Song P, Schrieber S, Liu Q, Xu Q, Blumenthal G, Amiri KL, Cortazar P, Ibrahim A, Justice R, Wang Y, Tang S, Booth B, Mehrotra N, Rahman A (2014) Exposure–response relationship of T-DM1: insight into dose optimization for patients with HER2-positive metastatic breast cancer. Clin Pharmacol Ther 95:558–564. https://doi.org/10.1038/clpt.2014.24
Zhu M, Tang R, Doshi S, Oliner KS, Dubey S, Jiang Y, Donehower RC, Iveson T, Loh EY, Zhang Y (2015) Exposure–response analysis of rilotumumab in gastric cancer: the role of tumour MET expression. Br J Cancer 112:429–437. https://doi.org/10.1038/bjc.2014.649
Tabernero J, Ohtsu A, Muro K, Van Cutsem E, Oh SC, Bodoky G et al (2017) Exposure–response analyses of ramucirumab from two randomized, phase III trials of second-line treatment for advanced gastric or gastroesophageal junction cancer. Mol Cancer Ther 16:2215–2222. https://doi.org/10.1158/1535-7163.MCT-16-0895
Cohn AL, Yoshino T, Heinemann V, Obermannova R, Bodoky G, Prausová J, Garcia-Carbonero R, Ciuleanu T, Garcia-Alfonso P, Portnoy DC, Van Cutsem E, Yamazaki K, Clingan PR, Polikoff J, Lonardi S, O’Brien LM, Gao L, Yang L, Ferry D, Nasroulah F, Tabernero J (2017) Exposure–response relationship of ramucirumab in patients with advanced second-line colorectal cancer: exploratory analysis of the RAISE trial. Cancer Chemother Pharmacol 80:599–608. https://doi.org/10.1007/s00280-017-3380-z
Ajani JA, Udrea A, Sarosiek T, Shenker M, Morgan C, Pikiel J, Wojcik E, Swinson D, Joseph M, Luft A, Salek T, Tournigand C, Ferry D, Zhang Y, Long A, Kuo W-L, Gao L, Kauh J, Mansoor W (2017) A dose-response study of ramucirumab treatment in patients with gastric cancer/gastroesophageal junction adenocarcinoma: Primary results of 4 dosing regimens in the phase 2 trial I4T-MC-JVDB. Ann Oncol 28(suppl 5):abstr 698P
O’Brien L, Westwood P, Gao L, Heathman M (2017) Population pharmacokinetic meta-analysis of ramucirumab in cancer patients. Br J Clin Pharmacol 83:2741–2751. https://doi.org/10.1111/bcp.13403
Yang J, Zhao H, Garnett C, Rahman A, Gobburu JV, Pierce W, Schechter G, Summers J, Keegan P, Booth B, Wang Y (2013) The combination of exposure–response and case–control analyses in regulatory decision making. J Clin Pharmacol 53:160–166. https://doi.org/10.1177/0091270012445206
Rubin D (1980) Bias reduction using Mahalanobis metric matching. Biometrics 36:293–298
Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C, Safran H, dos Santos LV, Aprile G, Ferry DR, Melichar B, Tehfe M, Topuzov E, Zalcberg JR, Chau I, Campbell W, Sivanandan C, Pikiel J, Koshiji M, Hsu Y, Liepa AM, Gao L, Schwartz JD, Tabernero J (2014) Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 383:31–39. https://doi.org/10.1016/S0140-6736(13)61719-5
Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, Hironaka S, Sugimoto N, Lipatov O, Kim TY, Cunningham D, Rougier P, Komatsu Y, Ajani J, Emig M, Carlesi R, Ferry D, Chandrawansa K, Schwartz JD, Ohtsu A (2014) Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol 15:1224–1235. https://doi.org/10.1016/S1470-2045(14)70420-6
Herbst RS, Sun Y, Eberhardt WE, Germonpre P, Saijo N, Zhou C, Wang J, Li L, Kabbinavar F, Ichinose Y, Qin S, Zhang L, Biesma B, Heymach JV, Langmuir P, Kennedy SJ, Tada H, Johnson BE (2010) Vandetanib plus docetaxel versus docetaxel as second-line treatment for patients with advanced non-small-cell lung cancer (ZODIAC): a double-blind, randomised, phase 3 trial. Lancet Oncol 11:619–626. https://doi.org/10.1016/S1470-2045(10)70132-7
Jin R, Li H, Zhang LH, Zhao H, Fashoyin-Aje L, Lemery S, Keegan P, Booth B, Atiqur Rahman N, Wang Y, Sinha V, Zhao L (2015) Exposure–response (E–R) and case–control analyses of ramucirumab leading to recommendation for dosing optimization in patients with gastric cancer. J Clin Oncol 33(suppl):abstr 2578. https://doi.org/10.1200/jco.2015.33.15_suppl.2578
Casak SJ, Fashoyin-Aje I, Lemery SJ, Zhang L, Jin R, Li H, Zhao L, Zhao H, Zhang H, Chen H, He K, Dougherty M, Novak R, Kennett S, Khasar S, Helms W, Keegan P, Pazdur R (2015) FDA approval summary: ramucirumab for gastric cancer. Clin Cancer Res 21:3372–3376. https://doi.org/10.1158/1078-0432.CCR-15-0600
Acknowledgements
We thank the patients, their families, the study sites, and the study personnel who participated in this clinical trial. Eli Lilly and Company contracted with Syneos Health for writing support provided by Andrea D. Humphries, PhD, and Ira Ayene, PhD, and editing support provided by Angela C. Lorio, ELS.
Funding
Funded by: Eli Lilly and Company.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Ling Gao, Lisa M. O’Brien, Pablo Lee, Annamaria Zimmermann, David R. Ferry, and Allen S. Melemed are full-time employees and stockholders of Eli Lilly and Company. Egbert F. Smit has received honoraria from Eli Lilly and Company. Edward B. Garon received research funding from AstraZeneca, Bristol-Myers Squibb, Genentech, Merck, Mirati, Pfizer, Novartis, Boehringer Ingelheim, and Eli Lilly and Company. Martin Reck has received honoraria for lectures and consultancy from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Eli Lilly and Company, Merck, Merck Sharp & Dohme, Novartis, Pfizer, and Roche/Genentech. Federico Cappuzzo has received consultancy fees from Eli Lilly and Company. Maurice Pérol has received honoraria as a consultant/advisory board member for Eli Lilly and Company. Paolo Bidoli is on the advisory board for Boehringer Ingelheim, Bristol-Myers Squibb, and Eli Lilly and Company. Roger B. Cohen declares grant support to his institution from ImClone. This study is funded by Eli Lilly and Company.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Smit, E.F., Garon, E.B., Reck, M. et al. Exposure–response relationship for ramucirumab from the randomized, double-blind, phase 3 REVEL trial (docetaxel versus docetaxel plus ramucirumab) in second-line treatment of metastatic non-small cell lung cancer. Cancer Chemother Pharmacol 82, 77–86 (2018). https://doi.org/10.1007/s00280-018-3560-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-018-3560-5