Abstract
Purpose
Fluorouracil monotherapy, instead of the FOLFOX or FOLFIRI regimen, is administered to patients intolerant to oxaliplatin or irinotecan because of their adverse effects. A prospective clinical trial was designed to evaluate the efficacy and safety of fluorouracil monotherapy combined with panitumumab administered to patients with KRAS wild-type (WT) metastatic colorectal cancer (mCRC) intolerant to oxaliplatin and irinotecan. Screening for potential serum biomarkers to predict early therapeutic responses was conducted.
Methods
This single-arm, open-label multicenter phase II trial recruited patients with KRAS WT mCRC from 16 institutes between January 2012 and October 2014. Panitumumab (6 mg/kg) was intravenously administered every 2 weeks, combined with fluorouracil monotherapy, in 2-week cycles. The primary objective was overall response rate, and secondary endpoints included disease-control rate, progression-free survival, overall survival, toxicity, and blood-test data.
Results
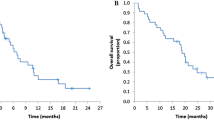
Forty patients (male, 65.0%; median age, 74 years; colon cancer, 72.5%) met eligibility criteria and received 7 cycles (median) of fluorouracil chemotherapy combined with panitumumab. There were no treatment-related deaths. Median time to treatment failure was 3.2 months. 23 (57.5%) patients experienced at least one adverse effect ≥ grade 3. The response rate was 10.0% (95% confidence interval 2.8–23.7%). Median progression-free survival and overall survival were 4.3 and 11.3 months, respectively. Total lactase dehydrogenase (LDH) levels and those of LDH-4 and LDH-5, quickly changed with disease reduction or progression.
Conclusions
Fluorouracil monotherapy combined with panitumumab was safely administered to patients with KRAS WT mCRC intolerant to oxaliplatin and irinotecan. Serum LDH levels may predict early responses.


Similar content being viewed by others
References
Brenner H, Kloor M, Pox CP (2014) Colorectal cancer. Lancet 383(9927):1490–1502
Primrose J, Falk S, Finch-Jones M, Valle J, O’Reilly D, Siriwardena A, Hornbuckle J, Peterson M, Rees M, Iveson T, Hickish T, Butler R, Stanton L, Dixon E, Little L, Bowers M, Pugh S, Garden OJ, Cunningham D, Maughan T, Bridgewater J (2014) Systemic chemotherapy with or without cetuximab in patients with resectable colorectal liver metastasis: the New EPOC randomised controlled trial. Lancet Oncol 15(6):601–611
Denda T, Kanda M, Morita Y, Kim HM, Kashiwada T, Matsuda C, Fujieda S, Nakata K, Murotani K, Oba K, Sakamoto J, Mishima H (2016) Pharmacokinetic dose adjustment of 5-FU in modified FOLFOX7 plus bevacizumab for metastatic colorectal cancer in Japanese patients: a-JUST phase II clinical trial. Cancer Chemother Pharmacol 78(6):1253–1261
Maughan TS, Adams RA, Smith CG, Meade AM, Seymour MT, Wilson RH, Idziaszczyk S, Harris R, Fisher D, Kenny SL, Kay E, Mitchell JK, Madi A, Jasani B, James MD, Bridgewater J, Kennedy MJ, Claes B, Lambrechts D, Kaplan R, Cheadle JP (2011) Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet 377(9783):2103–2114
Kataoka M, Kanda M, Ishigure K, Matsuoka H, Sato Y, Takahashi T, Tanaka C, Deguchi T, Shibata Y, Sato M, Inagaki H, Matsui T, Kondo A, Takano N, Tanaka H, Sakamoto J, Oba K, Kondo K (2017) The COMET Open-label Phase II Study of neoadjuvant FOLFOX or XELOX treatment combined with molecular targeting monoclonal antibodies in patients with resectable liver metastasis of colorectal cancer. Ann Surg Oncol 24(2):546–553
Beumer JH, Chu E, Salamone SJ (2012) Body-surface area-based chemotherapy dosing: appropriate in the 21st century? J Clin Oncol 30(31):3896–3897
Heinemann V, von Weikersthal LF, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran SE, Heintges T, Lerchenmuller C, Kahl C, Seipelt G, Kullmann F, Stauch M, Scheithauer W, Hielscher J, Scholz M, Muller S, Link H, Niederle N, Rost A, Hoffkes HG, Moehler M, Lindig RU, Modest DP, Rossius L, Kirchner T, Jung A, Stintzing S (2014) FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol 15(10):1065–1075
Venook AND, Lenz H, Innocenti F, Mahoney M, O’Neil B, Shaw J, Polite B, Hochster H, Atkins J, Goldberg R, Mayer R, Schilsky R, Bertagnolli M, Blanke C (2014) CALGB/SWOG 80405: phase III trial of irinotecan/5-FU/leucovorin (FOLFIRI) or oxaliplatin/5-FU/leucovorin (mFOLFOX6) with bevacizumab (BV) or cetuximab (CET) for patients (pts) with KRAS wild-type (wt) untreated metastatic adenocarcinoma of the colon or rectum (MCRC). J Clin Oncol 32:5
Ducreux M, Bennouna J, Hebbar M, Ychou M, Lledo G, Conroy T, Adenis A, Faroux R, Rebischung C, Bergougnoux L, Kockler L, Douillard JY (2011) Capecitabine plus oxaliplatin (XELOX) versus 5-fluorouracil/leucovorin plus oxaliplatin (FOLFOX-6) as first-line treatment for metastatic colorectal cancer. Int J Cancer 128(3):682–690
Giantonio BJ, Catalano PJ, Meropol NJ, O’Dwyer PJ, Mitchell EP, Alberts SR, Schwartz MA, Benson AB 3rd (2007) Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol 25(12):1539–1544
Amado RG, Wolf M, Peeters M, Van Cutsem E, Siena S, Freeman DJ, Juan T, Sikorski R, Suggs S, Radinsky R, Patterson SD, Chang DD (2008) Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol 26(10):1626–1634
Douillard JY, Oliner KS, Siena S, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J, Rivera F, Kocakova I, Ruff P, Blasinska-Morawiec M, Smakal M, Canon JL, Rother M, Williams R, Rong A, Wiezorek J, Sidhu R, Patterson SD (2013) Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med 369(11):1023–1034
Buijsen J, van Stiphout RG, Menheere PP, Lammering G, Lambin P (2014) Blood biomarkers are helpful in the prediction of response to chemoradiation in rectal cancer: a prospective, hypothesis driven study on patients with locally advanced rectal cancer. Radiother Oncol 111(2):237–242
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247
Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J, Rivera F, Kocakova I, Ruff P, Blasinska-Morawiec M, Smakal M, Canon JL, Rother M, Oliner KS, Wolf M, Gansert J (2010) Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol 28(31):4697–4705
De Gramont A, Krulik M, Cady J, Lagadec B, Maisani JE, Loiseau JP, Grange JD, Gonzalez-Canali G, Demuynck B, Louvet C et al (1988) High-dose folinic acid and 5-fluorouracil bolus and continuous infusion in advanced colorectal cancer. Eur J Cancer Clin Oncol 24(9):1499–1503
Van Cutsem E, Twelves C, Cassidy J, Allman D, Bajetta E, Boyer M, Bugat R, Findlay M, Frings S, Jahn M, McKendrick J, Osterwalder B, Perez-Manga G, Rosso R, Rougier P, Schmiegel WH, Seitz JF, Thompson P, Vieitez JM, Weitzel C, Harper P (2001) Oral capecitabine compared with intravenous fluorouracil plus leucovorin in patients with metastatic colorectal cancer: results of a large phase III study. J Clin Oncol 19(21):4097–4106
Kwakman JJM, Simkens LHJ, van Rooijen JM, van de Wouw AJ, Ten Tije AJ, Creemers GJM, Hendriks MP, Los M, van Alphen RJ, Polee MB, Muller EW, van der Velden AMT, van Voorthuizen T, Koopman M, Mol L, van Werkhoven E, Punt CJA (2017) Randomized phase III trial of S-1 versus capecitabine in the first-line treatment of metastatic colorectal cancer: SALTO study by the Dutch Colorectal Cancer Group. Ann Oncol 28(6):1288–1293
Douillard JY, Hoff PM, Skillings JR, Eisenberg P, Davidson N, Harper P, Vincent MD, Lembersky BC, Thompson S, Maniero A, Benner SE (2002) Multicenter phase III study of uracil/tegafur and oral leucovorin versus fluorouracil and leucovorin in patients with previously untreated metastatic colorectal cancer. J Clin Oncol 20(17):3605–3616
Munemoto Y, Kanda M, Ishibashi K, Hata T, Kobayashi M, Hasegawa J, Fukunaga M, Takagane A, Otsuji T, Miyake Y, Nagase M, Sakamoto J, Matsuoka M, Oba K, Mishima H (2015) Capecitabine and oxaliplatin combined with bevacizumab are feasible for treating selected Japanese patients at least 75 years of age with metastatic colorectal cancer. BMC Cancer 15:786
Alonso de la Pena V, Dios PD, Rocamonde SL, Sierra RT, Rodriguez-Segade S (2004) A standardised protocol for the quantification of lactate dehydrogenase activity in saliva. Arch Oral Biol 49(1):23–27
Stintzing S, Modest DP, Rossius L, Lerch MM, von Weikersthal LF, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran SE, Heintges T, Lerchenmuller C, Kahl C, Seipelt G, Kullmann F, Stauch M, Scheithauer W, Held S, Giessen-Jung C, Moehler M, Jagenburg A, Kirchner T, Jung A, Heinemann V (2016) FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab for metastatic colorectal cancer (FIRE-3): a post-hoc analysis of tumour dynamics in the final RAS wild-type subgroup of this randomised open-label phase 3 trial. Lancet Oncol 17(10):1426–1434
Rivera F, Karthaus M, Hecht JR, Sevilla I, Forget F, Fasola G, Canon JL, Guan X, Demonty G, Schwartzberg LS (2017) Final analysis of the randomised PEAK trial: overall survival and tumour responses during first-line treatment with mFOLFOX6 plus either panitumumab or bevacizumab in patients with metastatic colorectal carcinoma. Int J Colorectal Dis 32(8):1179–1190
Price T, Kim TW, Li J, Cascinu S, Ruff P, Suresh AS, Thomas A, Tjulandin S, Guan X, Peeters M (2016) Final results and outcomes by prior bevacizumab exposure, skin toxicity, and hypomagnesaemia from ASPECCT: randomized phase 3 non-inferiority study of panitumumab versus cetuximab in chemorefractory wild-type KRAS exon 2 metastatic colorectal cancer. Eur J Cancer 68:51–59
Holch JW, Ricard I, Stintzing S, Modest DP, Heinemann V (2017) The relevance of primary tumour location in patients with metastatic colorectal cancer: a meta-analysis of first-line clinical trials. Eur J Cancer 70:87–98
Carrato A, Abad A, Massuti B, Gravalos C, Escudero P, Longo-Munoz F, Manzano JL, Gomez A, Safont MJ, Gallego J, Garcia-Paredes B, Pericay C, Duenas R, Rivera F, Losa F, Valladares-Ayerbes M, Gonzalez E, Aranda E (2017) First-line panitumumab plus FOLFOX4 or FOLFIRI in colorectal cancer with multiple or unresectable liver metastases: a randomised, phase II trial (PLANET-TTD). Eur J Cancer 81:191–202
Tie J, Kinde I, Wang Y, Wong HL, Roebert J, Christie M, Tacey M, Wong R, Singh M, Karapetis CS, Desai J, Tran B, Strausberg RL, Diaz LA Jr, Papadopoulos N, Kinzler KW, Vogelstein B, Gibbs P (2015) Circulating tumor DNA as an early marker of therapeutic response in patients with metastatic colorectal cancer. Ann Oncol 26(8):1715–1722
Silvestris N, Scartozzi M, Graziano G, Santini D, Lorusso V, Maiello E, Barni S, Cinieri S, Loupakis F, Pisconti S, Brunetti AE, Palasciano G, Palmieri VO, Del Prete M, Dell’Aquila E, Latiano TP, Petrelli F, Lutrino S, Rossini D, Giampieri R, Lotesoriere C, Cascinu S (2015) Basal and bevacizumab-based therapy-induced changes of lactate dehydrogenases and fibrinogen levels and clinical outcome of previously untreated metastatic colorectal cancer patients: a multicentric retrospective analysis. Expert Opin Biol Ther 15(2):155–162
Giatromanolaki A, Sivridis E, Gatter KC, Turley H, Harris AL, Koukourakis MI (2006) Lactate dehydrogenase 5 (LDH-5) expression in endometrial cancer relates to the activated VEGF/VEGFR2 (KDR) pathway and prognosis. Gynecol Oncol 103(3):912–918
Inanc M, Er O, Karaca H, Berk V, Ozkan M, Dikilitas M, Elmali F (2013) D-dimer is a marker of response to chemotherapy in patients with metastatic colorectal cancer. J buon 18(2):391–397
Goodwin ML, Gladden LB, Nijsten MW, Jones KB (2014) Lactate and cancer: revisiting the warburg effect in an era of lactate shuttling. Front Nutr 1:27
Sanchez-Sanchez AM, Antolin I, Puente-Moncada N, Suarez S, Gomez-Lobo M, Rodriguez C, Martin V (2015) Melatonin cytotoxicity is associated to warburg effect inhibition in ewing sarcoma cells. PLoS ONE 10(8):e0135420
Bouafia F, Drai J, Bienvenu J, Thieblemont C, Espinouse D, Salles G, Coiffier B (2004) Profiles and prognostic values of serum LDH isoenzymes in patients with haematopoietic malignancies. Bull Cancer 91(7–8):E229–E240
Patel PS, Adhvaryu SG, Balar DB (1994) Serum lactate dehydrogenase and its isoenzymes in leukemia patients: possible role in diagnosis and treatment monitoring. Neoplasma 41(1):55–59
Zhou GQ, Ren XY, Mao YP, Chen L, Sun Y, Liu LZ, Li L, Lin AH, Mai HQ, Ma J (2016) Prognostic implications of dynamic serum lactate dehydrogenase assessments in nasopharyngeal carcinoma patients treated with intensity-modulated radiotherapy. Sci Rep 6:22326
Neal JL, Lowe NK, Corwin EJ (2013) Serum lactate dehydrogenase profile as a retrospective indicator of uterine preparedness for labor: a prospective, observational study. BMC Pregnancy Childbirth 13:128
Scartozzi M, Giampieri R, Maccaroni E, Del Prete M, Faloppi L, Bianconi M, Galizia E, Loretelli C, Belvederesi L, Bittoni A, Cascinu S (2012) Pre-treatment lactate dehydrogenase levels as predictor of efficacy of first-line bevacizumab-based therapy in metastatic colorectal cancer patients. Br J Cancer 106(5):799–804
Marmorino F, Salvatore L, Barbara C, Allegrini G, Antonuzzo L, Masi G, Loupakis F, Borelli B, Chiara S, Banzi MC, Miraglio E, Amoroso D, Dargenio F, Bonetti A, Martignetti A, Paris M, Tomcikova D, Boni L, Falcone A, Cremolini C (2017) Serum LDH predicts benefit from bevacizumab beyond progression in metastatic colorectal cancer. Br J Cancer 116(3):318–323
Kovesi TA, Hsu E (1994) Changes in lactate dehydrogenase isoenzymes associated with relapse of childhood acute lymphocytic leukemia. Pediatr Hematol Oncol 11(5):527–533
Nagai Y, Beppu T, Sakamoto Y, Miyamoto Y, Hayashi H, Nitta H, Imai K, Masuda T, Okabe H, Hirashima K, Imamura Y, Baba Y, Chikamoto A, Baba H (2014) Carcinoembryonic antigen half-life is an early predictor of therapeutic effects in induction chemotherapy for liver metastases from colorectal cancer. Anticancer Res 34(10):5529–5535
Rees M, Tekkis PP, Welsh FK, O’Rourke T, John TG (2008) Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg 247(1):125–135
Kazama Y, Watanabe T, Kanazawa T, Tanaka J, Tanaka T, Nagawa H (2007) Microsatellite instability in poorly differentiated adenocarcinomas of the colon and rectum: relationship to clinicopathological features. J Clin Pathol 60(6):701–704
Yokota T, Ura T, Shibata N, Takahari D, Shitara K, Nomura M, Kondo C, Mizota A, Utsunomiya S, Muro K, Yatabe Y (2011) BRAF mutation is a powerful prognostic factor in advanced and recurrent colorectal cancer. Br J Cancer 104(5):856–862
Funding
This study was supported, in part, by the nonprofit organization Epidemiological and Clinical Research Information Network (ECRIN).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Tadamichi Denda received research funding from Sanofi Co., Ltd., Boehringer Ingelheim Co., Ltd., and MSD Co., Ltd. Junichi Sakamoto serves as an advisor to Takeda Pharmaceutical Company Ltd. and received honoraria as lecture fees from Tsumura Co., Ltd., and from Chugai Pharmaceutical Co., Ltd. Hideyuki Mishima received lecture fees from Chugai Pharmaceutical Co., Ltd., and research funding from Chugai Pharmaceutical Co., Ltd. and Yakult Co., Ltd.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Kaplan–Meier curves of (a) progression-free and (b) overall survival according to treatment regimen. (TIF 5194 KB)
Rights and permissions
About this article
Cite this article
Munemoto, Y., Kanda, M., Oba, K. et al. A phase II trial to evaluate the efficacy of panitumumab combined with fluorouracil-based chemotherapy for metastatic colorectal cancer: the PF trial. Cancer Chemother Pharmacol 81, 829–838 (2018). https://doi.org/10.1007/s00280-018-3556-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-018-3556-1