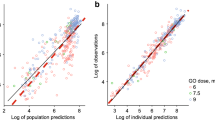
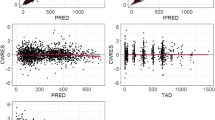
Abstract
Purpose
CPX-351 is a novel liposomal formulation of cytarabine and daunorubicin which has recently been FDA approved for treatment of acute myeloid leukemia (AML). The current study investigated the pharmacokinetics (PK) of this liposomal formulation.
Methods
CPX-351 PK data (cytarabine, daunorubicin, and metabolites) from a phase I study of relapsed and refractory AML were used for the analysis. Therapy was given days 1, 3, and 5 of induction (3–134 units/m2). We developed a population PK model to characterize CPX-351 disposition.
Results
39 patients (3589 samples) were evaluated. Liposomal cytarabine and daunorubicin were modeled separately with their respective metabolites. A one-compartment model fit the parent compounds well; the metabolites required two-compartment models. Weight was an independent predictor of liposomal volumes; mild renal and liver dysfunction were not predictors of clearance or volume (maximum creatinine 1.6 mg/dL and total bilirubin 1.8 mg/dL). Liposomal clearances of the two drugs were highly correlated and 1000-fold smaller than published non-encapsulated values supporting prolonged encapsulation in the liposome.
Conclusions
The PK model demonstrates prolonged exposure to cytarabine and daunorubicin without increases in non-hematologic toxicity that indicates retention of the drugs within the liposome. The unique pharmacology of this formulation may allow for simplified regimens and improved outcomes.



Similar content being viewed by others
References
Cashen A, VanTine BA (2012) Hematology and oncology subspecialty consult, 3rd edn. Lippincott, Williams, and Wilkins, Philadelphia
Dohner H, Weisdorf DJ, Bloomfield CD (2015) Acute myeloid leukemia. N Engl J Med 373:1136–1152
Rahman AM, Yusuf SW, Ewer MS (2007) Anthracycline-induced cardiotoxicity and the cardiac-sparing effect of liposomal formulation. Int J Nanomed 2:567–583
Drummond DC, Meyer O, Hong K et al (1999) Optimizing liposomes for delivery of chemotherapeutic agents to solid tumors. Pharmacol Rev 51:691–743
Mayer LD, Harasym TO, Tardi PG et al (2006) Ratiometric dosing of anticancer drug combinations: controlling drug ratios after systemic administration regulates therapeutic activity in tumor-bearing mice. Mol Cancer Ther 5:1854–1863
Tardi P, Johnstone S, Harasym N et al (2009) In vivo maintenance of synergistic cytarabine:daunorubicin ratios greatly enhances therapeutic efficacy. Leuk Res 33:129–139
Lancet JE, Cortes JE, Hogge DE et al (2014) Phase 2 trial of CPX-351, a fixed 5:1 molar ratio of cytarabine/daunorubicin, vs cytarabine/daunorubicin in older adults with untreated AML. Blood 123:3239–3246
Cortes JE, Goldberg SL, Feldman EJ et al (2015) Phase II, multicenter, randomized trial of CPX-351 (cytarabine:daunorubicin) liposome injection versus intensive salvage therapy in adults with first relapse AML. Cancer 121:234–242
VYXEOS™ (daunorubicin and cytarabine) liposome for injection, for intravenous use (2017) https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/209401s000lbl.pdf. Accessed 21 Oct 2017
Feldman EJ, Lancet JE, Kolitz JE et al (2011) First-in-man study of CPX-351: a liposomal carrier containing cytarabine and daunorubicin in a fixed 5:1 molar ratio for the treatment of relapsed and refractory acute myeloid leukemia. J Clin Oncol 29:979–985
Radeski D, Cull GM, Cain M et al (2011) Effective clearance of Ara-U the major metabolite of cytosine arabinoside (Ara-C) by hemodialysis in a patient with lymphoma and end-stage renal failure. Cancer Chemother Pharmacol 67:765–768
Galettis P (1996) Daunorubicin kinetics and drug resistance in leukaemia. University of Technology, Sydney
Koren G, Beatty K, Seto A et al (1992) The effects of impaired liver function on the elimination of antineoplastic agents. Ann Pharmacother 26:363–371
Krogh-Madsen M, Bender B, Jensen MK et al (2012) Population pharmacokinetics of cytarabine, etoposide, and daunorubicin in the treatment for acute myeloid leukemia. Cancer Chemother Pharmacol 69:1155–1163
Daunorubicin Hydrochloride Injection (2013) http://docs.boehringer-ingelheim.com/Prescribing%20Information/PIs/Ben%20Venue_Bedford%20Labs/55390–108-01%20DNOP_AQ%2020mg/5539010801. Accessed 21 Oct 2017
Gemtuzumab Ozogamicin Package Insert (2017) https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761060lbl.pdf. Accessed 21 Oct 2017
Midostaurin Package Insert (2017) https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/207997s000lbl.pdf. Accessed 21 Oct 2017
Enasidenib Package Insert (2017) https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/209606Orig1s000Lbl.pdf. Accessed 21 Oct 2017
Feldman EJ, Kolitz JE, Trang JM et al (2012) Pharmacokinetics of CPX-351; a nano-scale liposomal fixed molar ratio formulation of cytarabine:daunorubicin, in patients with advanced leukemia. Leuk Res 36:1283–1289
Thompson P, Wheeler HE, Delaney SM et al (2014) Pharmacokinetics and pharmacogenomics of daunorubicin in children: a report from the Children’s Oncology Group. Cancer Chemother Pharmacol 74:831–838
Lubieniecka JM, Graham J, Heffner D et al (2013) A discovery study of daunorubicin induced cardiotoxicity in a sample of acute myeloid leukemia patients prioritizes P450 oxidoreductase polymorphisms as a potential risk factor. Front Genet 4:231
Fernandez HF, Sun Z, Yao X et al (2009) Anthracycline dose intensification in acute myeloid leukemia. N Engl J Med 361:1249–1259
Lowenberg B, Pabst T, Vellenga E et al (2011) Cytarabine dose for acute myeloid leukemia. N Engl J Med 364:1027–1036
Lancet JE, Uy GL, Cortes JE et al (2016) Final results of a phase III randomized trial of CPX-351 versus 7 + 3 in older patients with newly diagnosed high risk (secondary) AML. American Society of Clinical Oncology. Chicago
Acknowledgements
The phase 1 clinical trial was funded by Celator Pharmaceuticals, Inc., a subsidiary of Jazz Pharmaceuticals, plc. Dr. Nikanjam received salary support from a National Institutes of Health grant (4T32HL066992—Academic Training in Hematology) and a Tower Cancer Research Foundation Career Development Award. Additional funding support was provided by a Research in Pediatric and Developmental Pharmacology NIH Grant (1U54HD090259-01, Dr. Capparelli) and the Aramont Foundation for the study of Hematological Malignancies (Dr. Schiller).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Capparelli serves on the data safety and monitoring board for Cempra Pharmaceuticals and The Medicines Company and has received consulting fees from Aptose, Plexxikon, Atox Bio Ltd, Celltrion, and Patara. Dr. Schiller has received research funding from Celator Pharmaceuticals. Dr. Louie is employed by Celator Pharmaceuticals.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nikanjam, M., Capparelli, E.V., Lancet, J.E. et al. Persistent cytarabine and daunorubicin exposure after administration of novel liposomal formulation CPX-351: population pharmacokinetic assessment. Cancer Chemother Pharmacol 81, 171–178 (2018). https://doi.org/10.1007/s00280-017-3484-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-017-3484-5