Abstract
Purpose
Accumulating evidence suggests that response-related parameters such as depth of response (DpR) might be associated with survival in colorectal cancer, which has not been shown in gastric cancer. This study aimed to evaluate whether DpR was associated with clinical outcomes in HER2-positive AGC patients treated with trastuzumab-based chemotherapy.
Methods
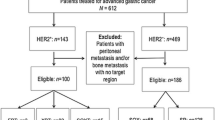
Fifty-seven HER2-positive AGC patients who were treated with trastuzumab in combination with fluoropyrimidines plus cisplatin therapy as first-line treatment were retrospectively enrolled. DpR was defined as the percent maximal tumor shrinkage of target lesions observed at the lowest point compared with baseline. The cutoff DpR level to discriminate better survival was based on receiver-operating characteristic curve analysis. Association of DpR with progression-free survival (PFS) and overall survival (OS) was assessed using the multivariable Cox proportional hazards model.
Results
Median DpR level was 56.8% (range −37.9 to 100%). In multivariate models adjusted for relevant variables, DpR, as a dichotomized variable with a cutoff level of 50% and a continuous variable, was significantly associated with PFS (hazard ratio [HR] 0.39 and 0.97; 95% confidence interval [CI] 0.22–0.68 and 0.96–0.98) and OS (HR 0.38 and 0.98; 95% CI 0.21–0.70 and 0.97–0.99). Clinically meaningful differences in PFS (median, 9.8 vs. 4.1 months; p < 0.001) and OS (median, 24.7 vs. 12.8 months; p < 0.001) were observed between the high DpR (≥50%) and the low DpR groups (<50%).
Conclusions
Higher DpR predicted favorable outcomes following trastuzumab-based chemotherapy in HER2-positive AGC patients.

Similar content being viewed by others
References
Jemal A, Bray F, Center MM et al (2011) Global cancer statistics. CA Cancer J Clin 61:69–90
Wagner AD, Unverzagt S, Grothe W et al (2010) Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev. doi:10.1002/14651858.CD004064.pub3
Van Cutsem E, Moiseyenko VM, Tjulandin S et al (2006) Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol 24:4991–4997
Koizumi W, Narahara H, Hara T et al (2008) S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol 9:215–221
Cunningham D, Starling N, Rao S et al (2008) Capecitabine and oxaliplatin for advanced esophagogastric cancer. New Eng J Med 358:36–46
Yarden Y, Sliwkowski MX (2001) Untangling the ErbB signalling network. Nat Rev Mol Cell Biol 2:127–137
Bang YJ, Van Cutsem E, Feyereislova A et al (2010) Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 376:687–697
Gomez-Martin C, Plaza JC, Pazo-Cid R et al (2013) Level of HER2 gene amplification predicts response and overall survival in HER2-positive advanced gastric cancer treated with trastuzumab. J Clin Oncol 31:4445–4452
Ock CY, Lee KW, Kim JW et al (2015) Optimal patient selection for trastuzumab treatment in HER2-positive advanced gastric cancer. Clin Cancer Res 21:2520–2529
Zhou J, Peng Z, Liu Y et al (2015) Predictive value of serum HER2 ECD in patients with HER2-positive advanced gastric cancer treated with trastuzumab plus chemotherapy. J Gastroenterol 50:955–961
Takahashi N, Furuta K, Taniguchi H et al (2016) Serum level of hepatocyte growth factor is a novel marker of predicting the outcome and resistance to the treatment with trastuzumab in HER2-positive patients with metastatic gastric cancer. Oncotarget 7:4925–4938
Paoletti X, Oba K, Bang YJ et al (2013) Progression-free survival as a surrogate for overall survival in advanced/recurrent gastric cancer trials: a meta-analysis. J Natl Cancer Inst 105:1667–1670
Cremolini C, Loupakis F, Antoniotti C et al (2015) Early tumor shrinkage and depth of response predict long-term outcome in metastatic colorectal cancer patients treated with first-line chemotherapy plus bevacizumab: results from phase III TRIBE trial by the Gruppo Oncologico del Nord Ovest. Ann Oncol 26:1188–1194
Heinemann V, Stintzing S, Modest DP et al (2015) Early tumour shrinkage (ETS) and depth of response (DpR) in the treatment of patients with metastatic colorectal cancer (mCRC). Eur J Cancer 51:1927–1936
Stintzing S, Modest DP, Rossius L et al (2016) FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab for metastatic colorectal cancer (FIRE-3): a post hoc analysis of tumour dynamics in the final RAS wild-type subgroup of this randomised open-label phase 3 trial. Lancet Oncol 17:1426–1434
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
Ruschoff J, Dietel M, Baretton G et al (2010) HER2 diagnostics in gastric cancer-guideline validation and development of standardized immunohistochemical testing. Virchows Arch 457:299–307
Hofmann M, Stoss O, Shi D et al (2008) Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology 52:797–805
Youden WJ (1950) Index for rating diagnostic tests. Cancer 3:32–35
Van Cutsem E, Bang YJ, Feng-Yi F et al (2015) HER2 screening data from ToGA: targeting HER2 in gastric and gastroesophageal junction cancer. Gastric Cancer 18:476–484
Sheffield BS, Garratt J, Kalloger SE et al (2014) HER2/neu testing in gastric cancer by immunohistochemistry: assessment of interlaboratory variation. Arch Pathol Lab Med 138:1495–1502
Kaga Y, Sunakawa Y, Kubota Y et al (2016) Early tumor shrinkage as a predictor of favorable outcomes in patients with advanced pancreatic cancer treated with FOLFIRINOX. Oncotarget 7:67314–67320
Boku N, Yamamoto S, Fukuda H et al (2009) Fluorouracil versus combination of irinotecan plus cisplatin versus S-1 in metastatic gastric cancer: a randomised phase 3 study. Lancet Oncol 10:1063–1069
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was approved by the ethics committees of Aichi Cancer Center Hospital and Tsuchiura Kyodo General Hospital and conducted in accordance with the 1964 Helsinki declaration and its later amendments. For this type of study, formal consent is not required.
Rights and permissions
About this article
Cite this article
Kadowaki, S., Masuishi, T., Eto, T. et al. Depth of response predicts the clinical outcome of advanced HER2-positive gastric cancer to trastuzumab-based first-line chemotherapy. Cancer Chemother Pharmacol 80, 807–813 (2017). https://doi.org/10.1007/s00280-017-3422-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-017-3422-6