Abstract
Purpose
This study utilizes FLT PET/CT imaging to characterize changes in tumor cell proliferation and vasculature during intermittent treatment with VEGR-TKI axitinib.
Methods
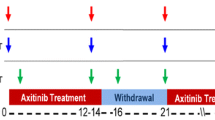
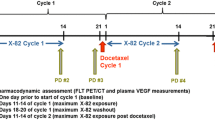
Patients with metastatic solid malignancies underwent 3-week treatment cycles with axitinib (7 and 5 mg BID for safety and pharmacodynamic cohorts, respectively). Cycles consisted of 2 weeks of treatment (dosing period) followed by a 1-week treatment break (washout period). Patients in the pharmacodynamic cohort had up to six FLT PET/CT scans (three scans in each cycle 1 and cycle 3) and had plasma VEGF concentrations measured at imaging timepoints. Changes in tumor SUVs and VEGF within and across drug cycles were investigated.
Results
Eight patients enrolled in the safety cohort where it was determined 7 mg axitinib was not tolerable due to severe adverse events, including three patients who experienced significant hypertension and thrombovascular effects. Sixteen patients enrolled in the pharmacodynamic cohort demonstrated significant decreases in SUVs and increases in VEGF during dosing periods. This was followed by significant increases in SUVs and decreases in VEGF during drug washout periods. No significant differences in SUVs or VEGF were found when comparing cycle 1 with cycle 3. A mixed effects model demonstrated significant negative correlation between SUV and VEGF.
Conclusions
Response to axitinib included diminished FLT uptake during dosing periods followed by increased FLT uptake during drug washout periods. These changes were not different when comparing treatment cycle 1 versus cycle 3, suggesting that the pharmacodynamic effect of intermittent axitinib is similar across multiple drug cycles.



Similar content being viewed by others
References
Folkman J (2003) Angiogenesis inhibitors: a new class of drugs. Cancer Biol Ther 2:S127–S133
Vasudev NS, Reynolds AR (2014) Anti-angiogenic therapy for cancer: current progress, unresolved questions and future directions. Angiogenesis 17:471–494
Bautch V (2012) VEGF-directed blood vessel patterning: from cells to organisms. In: Klagsburn M, D'Amore P (eds) Angiogensis biology and pathology. Cold Spring Harbor, New York, pp 29–40
Sonpavde G, Hutson T, Rini B (2008) Axitinib for renal cell carcinoma. Expert Opin Investig Drugs 17(5):741–748
Rini BI, Garrett M, Poland B, Dutcher JP, Rixe O, Wilding G et al (2013) Axitinib in metastatic renal cell carcinoma: results of a pharmacokinetic and pharmacodynamic analysis. J Clin Pharamcol 53(5):491–504
Hoh CK, Burris HA, Bendell JC, Tarazi J, Rosbrook B, Kim S, Infante JR, Reid TR (2014) Intermittent dosing of axitinib combined with chemotherapy is supported by 18FLT-PET in gastrointestinal tumours. Br J Cancer 110(4):875–881
Scagliotti G, Novello S, von Pawel J, Reck M, Pereira JR, Thomas M, Abrão Miziara JE, Balint B, De Marinis F, Keller A, Arén O, Csollak M, Albert I, Barrios CH, Grossi F, Krzakowski M, Cupit L, Cihon F, Dimatteo S, Hanna N (2010) Phase III study of carboplatin and paclitaxel alone or with sorafenib in advanced non-small-cell lung cancer. J Clin Oncol 28:1835–1842
Rugo H, Stopeck AT, Joy AA, Chan S, Verma S, Lluch A, Liau KF, Kim S, Bycott P, Rosbrook B, Bair AH, Soulieres D (2011) Randomized, placebo-controlled, double-blind, phase ii study of axitinib plus docetaxel versus docetaxel plus placebo in patients with metastatic breast cancer. J Clin Oncol 29(18):2459–2465
Kindler H, Ioka T, Richel DJ, Bennouna J, Létourneau R, Okusaka T, Funakoshi A, Furuse J, Park YS, Ohkawa S, Springett GM, Wasan HS, Trask PC, Bycott P, Ricart AD, Kim S, Van Cutsem E (2011) Axitinib plus gemcitabine versus placebo plus gemcitabine in patients with advanced pancreatic adenocarcinoma: a double-blind randomised phase 3 study. Lancet Oncol 12(3):256–262
Liu G, Jeraj R, Vanderhoek M, Perlman S, Kolesar J, Harrison M, Simoncic U, Eickhoff J, Carmichael L, Chao B, Marnocha R, Ivy P, Wilding G (2011) Pharmacodynamic study using FLT PET/CT in patients with renal cell cancer and other solid malignancies treated with sunitinib malate. Clin Cancer Res 17:7634–7644
Bruce J, Scully PC, Carmichael LL, Eickhoff JC, Perlman SB, Kolesar JM, Heideman JL, Jeraj R, Liu G (2015) Pharmacodynamic study of axitnib in patients with advanced malignancies assessed with 18F-3′deoxy-3′fluoro-l-thymidine positron emission tomorgraphy/computed tomography. Cancer Chemother Pharmacol 76(1):187–195
Shields A, Grierson J, Dohmen M, Machulla H, Stayanoff J, Lawhorn-Crews J, Obradovich J, Muzik O, Mangner T (1998) Imaging proliferation in vivo with [F-18]FLT and positron emission tomography. Nat Med 4:1334–1336
Yang W, Zhang Y, Fu Z, Sun X, Mu D, Yu J (2012) Imaging proliferation of 18F-FLT PET/CT correlated with the expression of microvessel density of tumour tissue in non-small-cell lung cancer. Eur J Nucl Med Mol Imaging 39:1289–1296
Horn K, Yap J, Agarwal N, Morton KA, Kadrmas DJ, Beardmore B, Butterfield RI, Boucher K, Hoffman JM (2015) FDG and FLT-PET for early measurment of response to 37.5 mg daily sunitinib therapy in metastatic renal cell carcinoma. Cancer Imaging 15:15
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
Bergers G, Hanahan D (2008) Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer 8(8):592–603
Acknowledgements
The authors would like to thank the nurses and research specialists of the UWCCC Phase I Program for their efforts in managing this trial, the WIMR PET imaging personnel for acquiring images, and the patients for their participation in this study.
Funding
This clinical trial was funded by a Pfizer Investigator-Initiated Research Grant (PI: Liu) (WS1610530) as well as a Prostate Cancer Foundation Young Investigator Award (MSN133051). This trial was conducted within the Cancer Therapy Discovery and Development (CTD2) research program with support from the Translational Imaging Research Working Group (TIRWG) at the UW Carbone Cancer Center.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to disclose.
Human and animal rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Matthew Scarpelli and Justine Yang Bruce are co-first authors and have contributed equally to the research and writing of the manuscript.
Rights and permissions
About this article
Cite this article
Scarpelli, M., Bruce, J.Y., Carmichael, L. et al. 18F-FLT PET/CT imaging in patients with advanced solid malignancies treated with axitinib on an intermittent dosing regimen. Cancer Chemother Pharmacol 78, 1245–1252 (2016). https://doi.org/10.1007/s00280-016-3183-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-016-3183-7