Abstract
Purpose
To establish the safety and pharmacokinetic profile of necitumumab in Japanese patients with advanced solid tumors not responsive to standard therapy or for which no standard therapy was available.
Methods
In this phase I study, patients aged ≥20 years with advanced solid tumors, and an Eastern Cooperative Oncology Group performance statuses of 0–1 were enrolled in a 3 + 3 design, with dose-escalation based on dose-limiting toxicity (DLT). Planned dose levels were: cohort 1: 600 mg IV, days 1 and 8, every 3 weeks; cohort 2: 800 mg IV, day 1, every 2 weeks; and cohort 3: 800 mg IV, days 1 and 8, every 3 weeks. After the first 6-week cycle, patients with an objective response or stable disease could continue to receive necitumumab (same dose and schedule) until disease progression or other withdrawal criteria were met. Safety, antitumor activity, and pharmacokinetics were assessed.
Results
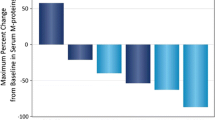
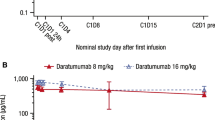
Fourteen of 15 enrolled patients received all scheduled infusions in cycle 1 (median cycles: N = 2, range 1–4). No DLTs were observed. The most common treatment-emergent adverse events were headache (73 %), dry skin (67 %), pruritus (60 %), and rash (53 %), mostly grade 1/2. All patients achieved serum trough concentrations >40 µg/mL, a level associated with antitumor activity in preclinical models. No patients had an objective response; stable disease was seen in 67 % of patients.
Conclusions
Necitumumab can be safety administered to Japanese patients at dose levels established in Western patients: 800 mg every 2 weeks, or on days 1 and 8 of a 3-week cycle.

Similar content being viewed by others
References
Zandi R, Larsen AB, Andersen P, Stockhausen MT, Poulsen HS (2007) Mechanisms for oncogenic activation of the epidermal growth factor receptor. Cell Signal 19(10):2013–2023. doi:10.1016/j.cellsig.2007.06.023
Pirker R, Pereira JR, Szczesna A, von Pawel J, Krzakowski M, Ramlau R, Vynnychenko I, Park K, Yu CT, Ganul V, Roh JK, Bajetta E, O’Byrne K, de Marinis F, Eberhardt W, Goddemeier T, Emig M, Gatzemeier U, Team FS (2009) Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): an open-label randomised phase III trial. Lancet 373(9674):1525–1531. doi:10.1016/S0140-6736(09)60569-9
Van Cutsem E, Kohne CH, Lang I, Folprecht G, Nowacki MP, Cascinu S, Shchepotin I, Maurel J, Cunningham D, Tejpar S, Schlichting M, Zubel A, Celik I, Rougier P, Ciardiello F (2011) Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol 29(15):2011–2019. doi:10.1200/JCO.2010.33.5091
Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J, Rivera F, Kocakova I, Ruff P, Blasinska-Morawiec M, Smakal M, Canon JL, Rother M, Oliner KS, Tian Y, Xu F, Sidhu R (2014) Final results from PRIME: randomized phase III study of panitumumab with FOLFOX4 for first-line treatment of metastatic colorectal cancer. Ann Oncol 25(7):1346–1355. doi:10.1093/annonc/mdu141
Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, Erfan J, Zabolotnyy D, Kienzer HR, Cupissol D, Peyrade F, Benasso M, Vynnychenko I, De Raucourt D, Bokemeyer C, Schueler A, Amellal N, Hitt R (2008) Platinum-based chemotherapy plus cetuximab in head and neck cancer. New Engl J Med 359(11):1116–1127. doi:10.1056/NEJMoa0802656
Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, Jones CU, Sur R, Raben D, Jassem J, Ove R, Kies MS, Baselga J, Youssoufian H, Amellal N, Rowinsky EK, Ang KK (2006) Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. New Engl J Med 354(6):567–578. doi:10.1056/NEJMoa053422
Dienstmann R, Felip E (2011) Necitumumab in the treatment of advanced non-small cell lung cancer: translation from preclinical to clinical development. Expert Opin Biol Ther 11(9):1223–1231. doi:10.1517/14712598.2011.595709
Li S, Kussie P, Ferguson KM (2008) Structural basis for EGF receptor inhibition by the therapeutic antibody IMC-11F8. Structure 16(2):216–227. doi:10.1016/j.str.2007.11.009
Samakoglu S, Deevi DS, Li H, Wang S, Murphy M, Bao C, Bassi R, Prewett M, Tonra JR (2012) Preclinical rationale for combining an EGFR antibody with cisplatin/gemcitabine for the treatment of NSCLC. Cancer Genom Proteom 9(2):77–92 (And data on file, ImClone Systems Incorporated)
Patel D, Saxena B, Zhou Q, Walton W, Srikakulum R, Manteiga R, Haurum J, Tonra JR, Kang X (2011) Differential induction of antibody-dependent cellular cytotoxicity (ADCC) against human EGFR-expressing NSCLC cell lines by necitumumab, cetuximab, and panitumumab. J Clin Oncol 29:(suppl; abstr e21075)
Kuenen B, Witteveen PO, Ruijter R, Giaccone G, Dontabhaktuni A, Fox F, Katz T, Youssoufian H, Zhu J, Rowinsky EK, Voest EE (2010) A phase I pharmacologic study of necitumumab (IMC-11F8), a fully human IgG1 monoclonal antibody directed against EGFR in patients with advanced solid malignancies. Clin Cancer Res 16(6):1915–1923. doi:10.1158/1078-0432.CCR-09-2425
Thatcher N, Hirsch FR, Luft AV, Szczesna A, Ciuleanu TE, Dediu M, Ramlau R, Galiulin RK, Balint B, Losonczy G, Kazarnowicz A, Park K, Schumann C, Reck M, Depenbrock H, Nanda S, Kruljac-Letunic A, Kurek R, Paz-Ares L, Socinski MA, investigators S (2015) Necitumumab plus gemcitabine and cisplatin versus gemcitabine and cisplatin alone as first-line therapy in patients with stage IV squamous non-small-cell lung cancer (SQUIRE): an open-label, randomised, controlled phase 3 trial. Lancet Oncol:(Epub June 1). doi:10.1016/S1470-2045(15)00021-2
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92(3):205–216
Paz-Ares L, Mezger J, Ciuleanu TE, Fischer JR, von Pawel J, Provencio M, Kazarnowicz A, Losonczy G, de Castro G Jr, Szczesna A, Crino L, Reck M, Ramlau R, Ulsperger E, Schumann C, Miziara JE, Lessa AE, Dediu M, Balint B, Depenbrock H, Soldatenkova V, Kurek R, Hirsch FR, Thatcher N, Socinski MA, Investigators I (2015) Necitumumab plus pemetrexed and cisplatin as first-line therapy in patients with stage IV non-squamous non-small-cell lung cancer (INSPIRE): an open-label, randomised, controlled phase 3 study. Lancet Oncol 16(3):328–337. doi:10.1016/S1470-2045(15)70046-X
Segaert S, Van Cutsem E (2005) Clinical signs, pathophysiology and management of skin toxicity during therapy with epidermal growth factor receptor inhibitors. Ann Oncol 16(9):1425–1433. doi:10.1093/annonc/mdi279
Shirao K, Yoshino T, Boku N, Kato K, Hamaguchi T, Yasui H, Yamamoto N, Tanigawara Y, Nolting A, Yoshino S (2009) A phase I escalating single-dose and weekly fixed-dose study of cetuximab pharmacokinetics in Japanese patients with solid tumors. Cancer Chemother Pharmacol 64(3):557–564. doi:10.1007/s00280-008-0904-6
Doi T, Ohtsu A, Tahara M, Tamura T, Shirao K, Yamada Y, Otani S, Yang BB, Ohkura M, Ohtsu T (2009) Safety and pharmacokinetics of panitumumab in Japanese patients with advanced solid tumors. Int J Clin Oncol 14(4):307–314. doi:10.1007/s10147-008-0855-2
Acknowledgments
The authors would like to acknowledge that Nancy Sheridan of INC Research, NC, USA provided writing assistance, which was funded by Eli Lilly and Company, Bridgewater, NJ.
Funding
This study was funded by Eli Lilly and Company.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Raffael Kurek and Sotaro Enatsu are employees of Eli Lilly and Company and its subsidiary Eli Lilly Japan K.K., respectively; they also have stock ownership as a part of bonus program.
Rights and permissions
About this article
Cite this article
Tamura, Y., Nokihara, H., Honda, K. et al. Phase I study of the second-generation, recombinant, human EGFR antibody necitumumab in Japanese patients with advanced solid tumors. Cancer Chemother Pharmacol 78, 995–1002 (2016). https://doi.org/10.1007/s00280-016-3154-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-016-3154-z