Abstract
Purpose
A capsule formulation of the tyrosine kinase inhibitor dovitinib (TKI258) was recently studied in a phase 3 renal cell carcinoma trial; however, tablets are the planned commercial formulation. Therefore, this randomized 2-way crossover study evaluated the bioequivalence of dovitinib tablet and capsule formulations in pretreated patients with advanced solid tumors, excluding breast cancer.
Methods
In this 2-part study, eligible patients received dovitinib 500 mg once daily on a 5-days-on/2-days-off schedule. During the 2-period bioequivalence phase, patients received their initial formulation (capsule or tablet) for 3 weeks before being switched to the alternative formulation in the second period. Patients could continue to receive dovitinib capsules on the same dosing schedule during the post-bioequivalence phase.
Results
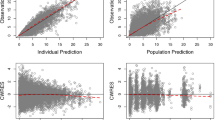
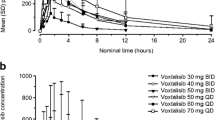
A total of 173 patients were enrolled into the bioequivalence phase of the study (capsule → tablet, n = 88; tablet → capsule, n = 85), and 69 patients had evaluable pharmacokinetics (PK) for both periods. PK analyses showed similar exposure and PK profiles for the dovitinib capsule and tablet formulations and supported bioequivalence [geometric mean ratios: AUClast, 0.95 (90 % CI 0.88–1.01); C max, 0.98 (90 % CI 0.91–1.05)]. The most common adverse events, suspected to be study drug related, included diarrhea (60 %), nausea (53 %), fatigue (45 %), and vomiting (43 %). Of 168 patients evaluable for response, 1 achieved a partial response, and stable disease was observed in 32 % of patients.
Conclusions
Dovitinib capsules and tablets were bioequivalent, with a safety profile similar to that observed in other dovitinib studies of patients with heavily pretreated advanced solid tumors.


Similar content being viewed by others
References
Schlessinger J (2000) Cell signaling by receptor tyrosine kinases. Cell 103:211–225
Vlahovic G, Crawford J (2003) Activation of tyrosine kinases in cancer. Oncologist 8:531–538
Dvorak HF (2003) Rous-whipple award lecture. how tumors make bad blood vessels and stroma. Am J Pathol 162:1747–1757
Man WY, Mak JP, Poon RY (2014) Dovitinib induces mitotic defects and activates the G2 DNA damage checkpoint. J Cell Mol Med 18:143–155
Huynh H, Chow PK, Tai WM, Choo SP, Chung AY, Ong HS, Soo KC, Ong R, Linnartz R, Shi MM (2012) Dovitinib demonstrates antitumor and antimetastatic activities in xenograft models of hepatocellular carcinoma. J Hepatol 56:595–601
Lee SH, Lopes de Menezes D, Vora J, Harris A, Ye H, Nordahl L, Garrett E, Samara E, Aukerman SL, Gelb AB, Heise C (2005) In vivo target modulation and biological activity of CHIR-258, a multitargeted growth factor receptor kinase inhibitor, in colon cancer models. Clin Cancer Res 11:3633–3641
Lamont FR, Tomlinson DC, Cooper PA, Shnyder SD, Chester JD, Knowles MA (2011) Small molecule FGF receptor inhibitors block FGFR-dependent urothelial carcinoma growth in vitro and in vivo. Br J Cancer 104:75–82
Angevin E, Lopez-Martin JA, Lin CC, Gschwend JE, Harzstark A, Castellano D, Soria JC, Sen P, Chang J, Shi M, Kay A, Escudier B (2013) Phase I study of dovitinib (TKI258), an oral FGFR, VEGFR, and PDGFR inhibitor, in advanced or metastatic renal cell carcinoma. Clin Cancer Res 19:1257–1268
Escudier B, Grunwald V, Ravaud A, Ou YC, Castellano D, Lin CC, Gschwend JE, Harzstark A, Beall S, Pirotta N, Squires M, Shi M, Angevin E (2014) Phase II results of dovitinib (TKI258) in patients with metastatic renal cell cancer. Clin Cancer Res 20:3012–3022
Kim KB, Chesney J, Robinson D, Gardner H, Shi MM, Kirkwood JM (2011) Phase I/II and pharmacodynamic study of dovitinib (TKI258), an inhibitor of fibroblast growth factor receptors and VEGF receptors, in patients with advanced melanoma. Clin Cancer Res 17:7451–7461
Scheid C, Reece D, Beksac M, Spencer A, Callander N, Sonneveld P, Kalimi G, Cai C, Shi M, Scott JW, Stewart AK (2012) A phase 2, multicenter, nonrandomized, open-label study of dovitinib (TKI258) in patients with relapsed or refractory multiple myeloma with or without t(4;14) translocation. ASH Annual Meeting Abstracts: Abstract 4055
André F, Bachelot T, Campone M, Dalenc F, Perez-Garcia JM, Hurvitz SA, Turner N, Rugo H, Smith JW, Deudon S, Shi M, Zhang Y, Kay A, Porta DG, Yovine A, Baselga J (2013) Targeting FGFR with dovitinib (TKI258): preclinical and clinical data in breast cancer. Clin Cancer Res 19:3693–3702
Konecny GE, Kolarova T, O’Brien NA, Winterhoff B, Yang G, Qi J, Qi Z, Venkatesan N, Ayala R, Luo T, Finn RS, Kristof J, Galderisi C, Porta DG, Anderson L, Shi MM, Yovine A, Slamon DJ (2013) Activity of the fibroblast growth factor receptor inhibitors dovitinib (TKI258) and NVP-BGJ398 in human endometrial cancer cells. Mol Cancer Ther 12:632–642
André F, Neven P, Musolino A, Latini L, Campone M, Cortes J, Barrios C, Squires M, Zhang Y, Deudon S, Gogov S, Blackwell K (2013) Dovitinib, a receptor tyrosine kinase inhibitor in combination with fulvestrant in postmenopausal endocrine resistant human epidermal growth factor receptor 2 negative (HER2-)/hormone receptor-positive (HR +) breast cancer: a phase II, randomized, double blind, placebo-controlled study. San Antonio Breast Cancer Symposium Abstracts: Abstract OT2-6-04
Sharma S, Britten CD, Mortimer J, Kulkarni S, Quinlan M, Liu A, Scott JW, George D (in press) The effect of formulation and food consumption on the bioavailability of dovitinib (TKI258) in patients with advanced solid tumors. Cancer Chemother Pharmacol
Sharma S, Ramanathan RK, George DJ, Quinlan M, Kulkarni S, Homsi A, Scott JW, Infante JR (2013) A randomized, crossover phase I study to assess the effects of formulation and meal consumption on the bioavailability of dovitinib (TKI258). J Clin Oncol 31:suppl; abstr 2593
Motzer RJ, Porta C, Vogelzang NJ, Sternberg CN, Szczylik C, Zolnierek J, Kollmannsberger C, Rha SY, Bjarnason GA, Melichar B, De Giorgi U, Grunwald V, Davis ID, Lee JL, Esteban E, Urbanowitz G, Cai C, Squires M, Marker M, Shi MM, Escudier B (2014) Dovitinib versus sorafenib for third-line targeted treatment of patients with metastatic renal cell carcinoma: an open-label, randomised phase 3 trial. Lancet Oncol 15:286–296
National Institutes of Health and National Cancer Institute (2009) National cancer institute common terminology criteria for adverse events version 4.0
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
Acknowledgments
This study was supported by Novartis Pharmaceuticals Corporation. Additional support was provided by the Institute for Drug Development, Cancer Therapy and Research Center at University of Texas Health Science Center San Antonio in San Antonio, TX Cancer Center Support Grant P30CA054174. We thank Peter J. Simon, Ph.D., and Amanda L. Kauffman, Ph.D., from ArticulateScience (funded by Novartis Pharmaceuticals Corporation) and Sudipta Ridhurkar from Novartis Healthcare Pvt Ltd for providing medical editorial assistance with this manuscript.
Funding
Funding for this study and editorial assistance for the manuscript was provided by Novartis Pharmaceuticals Corporation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sarantopoulos, J., Goel, S., Chung, V. et al. Randomized phase 1 crossover study assessing the bioequivalence of capsule and tablet formulations of dovitinib (TKI258) in patients with advanced solid tumors. Cancer Chemother Pharmacol 78, 921–927 (2016). https://doi.org/10.1007/s00280-016-3122-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-016-3122-7