Abstract
Purpose
Anti-epidermal growth factor receptor antibody therapy alone or in combination with irinotecan is recognized as a standard third-line treatment for KRAS wild-type unresectable metastatic colorectal cancer. However, in some cases, it is difficult to administer irinotecan after third-line treatment. Therefore, we examined the efficacy and safety of the combination of cetuximab and S-1 in patients with KRAS wild-type unresectable metastatic colorectal cancer who were previously treated with irinotecan, oxaliplatin, and fluoropyrimidines.
Methods
The study was designed as a phase II, non-randomized, open-label, multicenter trial. Cetuximab was initially administered at 400 mg/m2, followed by weekly infusion at 250 mg/m2. S-1 was administered at a fixed dose of 80 mg/m2 orally twice daily for 28 days followed by a 14-day break, resulting in a 6-week treatment course. The primary endpoint was progression-free survival (PFS). The secondary endpoints were the overall response rate (ORR), overall survival (OS), disease control rate (DCR), time to treatment failure, dose intensity, safety, and BRAF mutation status.
Results
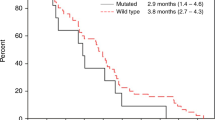
Thirty-seven patients were eligible. The median PFS was 5.5 months, the median OS was 13.5 months, the ORR was 29.7 %, and the DCR was 73.0 %. The relative dose intensity was 86.8 % for cetuximab and 88.1 % for S-1. Grade 3–4 adverse events that occurred in >10 % of the patient population included rash, dry skin, diarrhea, paronychia, anorexia, fatigue, mucositis, and neutropenia.
Conclusions
Combination therapy with cetuximab and S-1 was effective and well tolerated in patients with irinotecan-, oxaliplatin-, and fluoropyrimidine-refractory metastatic colorectal cancer.



Similar content being viewed by others
References
Kopetz S, Chang GJ, Overman MJ, Eng C, Sargent DJ, Larson DW, Grothey A, Vauthey JN, Nagorney DM, McWilliams RR (2009) Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol 27:3677–3683
Baselga J (2001) The EGFR as a target for anticancer therapy-focus on cetuximab. Eur J Cancer 37:S16–S22
Ciardiello F, Tortora G (2001) A novel approach in the treatment of cancer: targeting the epidermal growth factor receptor. Clin Cancer Res 7:2958–2970
Jonker DJ, O’Callaghan CJ, Karapetis CS, Zalcberg JR, Tu D, Au HJ, Berry SR, Krahn M, Price T, Simes RJ, Tebbutt NC, van Hazel G, Wierzbicki R, Langer C, Moore MJ (2007) Cetuximab for the treatment of colorectal cancer. N Engl J Med 357:2040–2048
Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C, Chau I, Van Cutsem E (2004) Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 351:337–345
Van Cutsem E, Köhne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, D’Haens G, Pintér T, Lim R, Bodoky G, Roh JK, Folprecht G, Ruff P, Stroh C, Tejpar S, Schlichting M, Nippgen J, Rougier P (2009) Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med 360:1408–1417
Bokemeyer C, Bondarenko I, Makhson A, Hartmann JT, Aparicio J, de Braud F, Donea S, Ludwig H, Schuch G, Stroh C, Loos AH, Zubel A, Koralewski P (2009) Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol 27:663–671
Karapetis CS, Khambata-Ford S, Jonker DJ, O’Callaghan CJ, Tu D, Tebbutt NC, Simes RJ, Chalchal H, Shapiro JD, Robitaille S, Price TJ, Shepherd L, Au HJ, Langer C, Moore MJ, Zalcberg JR (2008) K-ras Mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med 359:1757–1765
Nukatsuka M, Saito H, Nakagawa F, Tsujimoto H, Sakamoto K, Tsukioka S, Uchida J, Kiniwa M, Kobunai T, Takechi T (2012) Combination therapy using oral S-1 and targeted agents against human tumor xenografts in nude mice. Exp Ther Med 3:755–762
Overholser JP, Prewett MC, Hooper AT, Waksal HW, Hicklin DJ (2000) Epidermal growth factor receptor blockade by antibody IMC-C225 inhibits growth of a human pancreatic carcinoma xenograft in nude mice. Cancer 89:74–82
Kobunai T, Waranabe T, Fukusato T (2011) Antitumor activity of S-1 in combination with Cetuximab on human gastric cancer cell lines in vivo. Anticancer Res 31:3691–3696
Fukuda K, Saikawa Y, Takahashi M, Takahashi T, Wada N, Kawakubo H, Takeuchi H, Kitagawa Y (2012) Antitumor effect of cetuximab in combination with S-1 in EGFR-amplified gastric cancer cells. Int J Oncol 40:975–982
Shirasaka T (2009) Development history and concept of an oral anticancer agent S-1 (TS-1): its clinical usefulness and future vistas. Jpn J Clin Oncol 39:2–15
Amado RG, Wolf M, Peeters M, Van Cutsem E, Siena S, Freeman DJ, Juan T, Sikorski R, Suggs S, Radinsky R, Patterson SD, Chang DD (2008) Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol 26:1626–1634
Van Cutsem E, Peeters M, Siena S, Humblet Y, Hendlisz A, Neyns B, Canon JL, Van Laethem JL, Maurel J, Richardson G, Wolf M, Amado RG (2007) Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. J Clin Oncol 25:1658–1664
André T, Blons H, Mabro M, Chibaudel B, Bachet JB, Tournigand C, Bennamoun M, Artru P, Nguyen S, Ebenezer C, Aissat N, Cayre A, Penault-Llorca F, Laurent-Puig P, de Gramont A, GERCOR (2013) Panitumumab combined with irinotecan for patients with KRAS wild-type metastatic colorectal cancer refractory to standard chemotherapy: a GERCOR efficacy, tolerance, and translational molecular study. Ann Oncol 24:412–419
Shitara K, Yokota T, Takahari D, Shibata T, Ura T, Utsunomiya S, Inaba Y, Yamaura H, Sato Y, Najima M, Kawai H, Tajika M, Sawaki A, Yatabe Y, Muro K (2011) Phase II study of combination chemotherapy with irinotecan and cetuximab for pretreated metastatic colorectal cancer harboring wild-type KRAS. Invest New Drugs 29:688–693
Shitara K, Yuki S, Yoshida M, Takahari D, Utsunomiya S, Yokota T, Sato Y, Inaba Y, Tajika M, Kawai H, Yamaura H, Kato M, Yamazaki K, Komatsu Y, Muro K (2012) Phase II study of combination chemotherapy with biweekly cetuximab and irinotecan for wild-type KRAS metastatic colorectal cancer refractory to irinotecan, oxaliplatin, and fluoropyrimidines. Invest New Drugs 30:787–793
Tahara M, Shirao K, Boku N, Yamaguchi K, Komatsu Y, Inaba Y, Arai T, Mizunuma N, Satoh T, Takiuchi H, Nishina T, Sakata Y (2008) Multicenter phase II study of cetuximab plus irinotecan in metastatic colorectal carcinoma refractory to irinotecan, oxaliplatin and fluoropyrimidines. Jpn J Clin Oncol 38:762–769
Muro K, Yoshino T, Doi T, Shirao K, Takiuchi H, Hamamoto Y, Watanabe H, Yang BB, Asahi D (2009) A Phase 2 clinical trial of panitumumab monotherapy in Japanese patient as with metastatic Colorectal Cancer. Jpn J Clin Oncol 39:321–326
Sobrero AF, Maurel J, Fehrenbacher L, Scheithauer W, Abubakr YA, Lutz MP, Vega-Villegas ME, Eng C, Steinhauer EU, Prausova J, Lenz HJ, Borg C, Middleton G, Kröning H, Luppi G, Kisker O, Zubel A, Langer C, Kopit J, Burris HA 3rd (2008) EPIC: phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J Clin Oncol 26:2311–2319
Oki E, Emi Y, Miyamoto Y, Kabashima A, Higashi H, Ogata Y, Ikebe M, Saeki H, Tokunaga S, Shirabe K, Beppu T, Uchida S, Takatsuki M, Sakoda M, Eguchi S, Akagi Y, Kakeji Y, Baba H, Natsugoe S, Maehara Y, Kyushu Study Group of Clinical Cancer (KSCC) (2015) Phase II trial of S-1 and oxaliplatin plus cetuximab for colorectal cancer patients with initially unresectable or not optimally resectable liver metastases (KSCC1002). Ann Surg Oncol 22:S1067–S1074
Ogawa M, Anan T, Suzuki T, Okuma M, Ichihara K, Hasegawa T, Yoshida K, Yanaga K (2016) Initial report of phase II study on bi-weekly SOX plus cetuximab treatment for wild-type K-RAS advanced and recurrent colorectal cancer. Anticancer Res 36:2505–2511
Chuah B, Goh BC, Lee SC, Soong R, Lau F, Mulay M, Dinolfo M, Lim SE, Soo R, Furuie T, Saito K, Zergebel C, Rosen LS (2011) Comparison of the pharmacokinetics and pharmacodynamics of S-1 between Caucasian and East Asian patients. Cancer Sci 102:478–483
Acknowledgments
The KSCC0901 was conducted by the KSCC and Clinical Research Support Center (CReS) Kyushu. Merck Serono and Taiho Pharmaceutical Co. provided an unrestricted contribution to CReS Kyushu. We greatly thank the participated patients and their families. We are indebted to the physicians and all of the clinical study teams at the participating institutions. We thank all other co-medical staff, and the Independent Data Monitoring Committee (Kuniaki Shirao, Toshiro Kuroiwa, Yoichi Nakanishi, Shuji Nakano, and Eishi Baba) who contributed to this study. We also thank Ms. Taniguchi, Ms. Sakamoto, Ms. Shimamoto, Mr. Aratani, and the other staff at CReS Kyushu for their excellent collection and manage of data, secretarial assistance, and other support.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
H. Baba and K. Yoshida have received research grants from Merck Serono and Taiho Pharmaceutical Co., Ltd. T. Takahashi, E. Oki, A. Tsuji, H. Baba, K. Yoshida and Yoshihiko Maehara have received speaker honorarium from Merck Serono and Taiho Pharmaceutical Co., Ltd.. Y. Emi, K. Kobayashi, M. Shimokawa, T. Tanaka, Y. Akagi, Y. Ogata, S. Natsugoe declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Takahashi, T., Emi, Y., Oki, E. et al. Multicenter phase II study of combination therapy with cetuximab and S-1 in patients with KRAS exon 2 wild-type unresectable colorectal cancer previously treated with irinotecan, oxaliplatin, and fluoropyrimidines (KSCC 0901 study). Cancer Chemother Pharmacol 78, 585–593 (2016). https://doi.org/10.1007/s00280-016-3109-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-016-3109-4