Abstract
Purpose
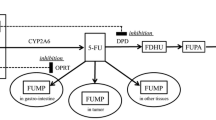
The options for improving the chemotherapeutic regimen consisting of bolus plus infusion of 5-fluorouracil (5-FU) include omitting the 5-FU bolus injection. We examined the effects of a 5-FU bolus injection on the activity of dihydropyrimidine dehydrogenase (DPD), which is the first and rate-limiting enzyme of 5-FU catabolism, in rats.
Methods
The rats were divided into three groups, and then continuous infusion (50 mg/m2/h) for 4 h was started with a bolus injection of saline, 20 mg/kg 5-FU, or 60 mg/kg 5-FU. Plasma 5-FU, uracil (Ura), dihydrouracil (UH2) levels, and hepatic DPD activity were determined after administration of 5-FU.
Results
The half-life after the end of the infusion (t 1/2, 4–8 h) of 5-FU in the rats given the bolus injection was significantly longer than in those that had been given saline, and it increased with increasing 5-FU bolus injection dosage (r = 0.801, p < 0.01). The plasma UH2/Ura ratio, an indirect biomarker of hepatic DPD activity, tended to be lower in the rats that had received a 5-FU bolus injection than in those that had not, and it remained low after infusion ended. The hepatic DPD activity in rats that had received a 5-FU bolus injection was significantly lower than in those that had not. Negative correlation was observed between DPD activity and bolus injection dosage (r = −0.691, p < 0.05).
Conclusions
A bolus injection suppresses hepatic DPD activity and its effects are dependent on dosage, resulting in slower elimination of 5-FU from the blood and contributing to long-term systemic exposure to 5-FU.




Similar content being viewed by others
References
Kochi M, Akiyama Y, Aoki T, Hagiwara K, Takahashi T, Hironaka K, Teranishi F, Osuka F, Takeuchi M, Fujii M, Nakajima T (2013) FOLFIRI plus bevacizumab as a first-line treatment for Japanese patients with metastatic colorectal cancer: a JACCRO CC-03 multicenter phase II study. Cancer Chemother Pharmacol 72:1097–1102
Saif MW, Choma A, Salamone SJ, Chu E (2009) Pharmacokinetically guided dose adjustment of 5-fluorouracil: a rational approach to improving therapeutic outcomes. J Natl Cancer Inst 101:1543–1552
Sakaeda T, Yamamori M, Kuwahara A, Nishiguchi K (2009) Pharmacokinetics and pharmacogenomics in esophageal cancer chemoradiotherapy. Adv Drug Deliv Rev 61:340–388
Tamura T, Kuwahara A, Kadoyama K, Yamamori M, Nishiguchi K, Inokuma T, Takemoto Y, Chayahara N, Okuno T, Miki I, Fujishima Y, Sakaeda T (2011) Effects of bolus injection of 5-fluorouracil on steady-state plasma concentrations of 5-fluorouracil in Japanese patients with advanced colorectal cancer. Int J Med Sci 8(5):406–412
Grothey A, Sargent D, Goldberg RM, Schmoll HJ (2004) Survival of patients with advanced colorectal cancer improves with the availability of fluorouracil-leucovorin, irinotecan, and oxaliplatin in the course of treatment. J Clin Oncol 22:1209–1214
Venook A (2005) Critical evaluation of current treatments in meta-static colorectal cancer. Oncologist 10:250–261
Lee JJ, Chu E (2007) An update on treatment advances for the first-line therapy of metastatic colorectal cancer. Cancer J 13:276–281
Sabharwal A, Kerr D (2007) Chemotherapy for colorectal cancer in the metastatic and adjuvant setting: past, present and future. Expert Rev Anticancer Ther 7:477–487
Noguchi C, Miyata H, Sato Y, Iwaki Y, Okuyama S (2011) Evaluation of bone toxicity in various bones of aged rats. J Toxicol Pathol 24(1):41–48
Diehl KH, Hull R, Morton D, Pfister R, Rabemampianina Y, Smith D, Vidal JM, van de Vorstenbosch C, European Federation of Pharmaceutical Industries Association and European Centre for the Validation of Alternative Methods (2001) A good practice guide to the administration of substances and removal of blood, including routes and volumes. J Appl Toxicol 21(1):15–23
Tateishi T, Nakura H, Watanabe M, TanakaM Kumai T, Kobayashi S (1996) Preliminary examination of the influence of incubation time or cytosolic protein concentration on dihydropyrimidine dehydrogenase activity. Clin Chim Acta 252:1–9
Kobuchi S, Kuwano S, Imoto K, Okada K, Nishimura A, Ito Y, Shibata N, Takada K (2013) A predictive biomarker for altered 5-fluorouracil pharmacokinetics following repeated administration in a rat model of colorectal cancer. Biopharm Drug Dispos 34(7):365–376
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Büchel B, Rhyn P, Schürch S, Bühr C, Amstutz U, Largiadèr CR (2013) LC-MS/MS method for simultaneous analysis of uracil, 5,6-dihydrouracil, 5-fluorouracil and 5-fluoro-5,6-dihydrouracil in human plasma for therapeutic drug monitoring and toxicity prediction in cancer patients. Biomed Chromatogr 27(1):7–16
Gamelin E, Boisdron-Celle M, Gu´erin-Meyer V, Delva R, Lortholary A, Genevieve F, Larra F, Ifrah N, Robert J (1999) Correlation between uracil and dihydrouracil plasma ratio, fluorouracil (5-FU) pharmacokinetic parameters, and tolerance in patients with advanced colorectal cancer: a potential interest for predicting 5-FU toxicity and determining optimal 5-FU dosage. J Clin Oncol 17(4):1105
Kobuchi S, Ito Y, Okada K, Imoto K, Kuwano S, Takada K (2013) Pharmacokinetic/pharmacodynamic modeling of 5-fluorouracil by using a biomarker to predict tumor growth in a rat model of colorectal cancer. J Pharm Sci 102(6):2056–2067
Kobuchi S, Ito Y, Okada K, Imoto K, Kuwano S, Takada K (2013) Pre-therapeutic assessment of plasma dihydrouracil/uracil ratio for predicting the pharmacokinetic parameters of 5-fluorouracil and tumor growth in a rat model of colorectal cancer. Biol Pharm Bull 36(6):907–916
Milano G, Chamorey AL (2002) Clinical pharmacokinetics of 5-fluorouracil with consideration of chronopharmacokinetics. Chronobiol Int 19(1):177–189
Kuwahara A, Yamamori M, Nishiguchi K, Okuno T, Chayahara N, Miki I, Tamura T, Kadoyama K, Inokuma T, Takemoto Y, Nakamura T, Kataoka K, Sakaeda T (2010) Effect of dose-escalation of 5-fluorouracil on circadian variability of its pharmacokinetics in Japanese patients with Stage III/IVa esophageal squamous cell carcinoma. Int J Med Sci. 7(1):48–54
Gamelin E, Delva R, Jacob J, Merrouche Y, Raoul JL, Pezet D, Dorval E, Piot G, Morel A, Boisdron-Celle M. Individual 5-fluorouracil dose adjustment based on pharmacokinetic follow-up compared with conventional dosage: results of a multicenter randomized trial in patients with metastatic colorectal cancer. J Clin Oncol 26(13):2099–2105
Acknowledgments
This study was supported in part by a Grant-in-Aid for Young Scientists (B) (No. 15K18937) from Ministry of Education, Culture, Sports, Science, and Technology (MEXT, Japan).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Kobuchi, S., Hayashi, A., Taniguchi, M. et al. Effects of a bolus injection of 5-fluorouracil on dihydropyrimidine dehydrogenase activity in rats receiving continuous infusion of 5-fluorouracil. Cancer Chemother Pharmacol 78, 517–523 (2016). https://doi.org/10.1007/s00280-016-3105-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-016-3105-8