Abstract
Purpose
To investigate the anticancer therapeutic potential of a new synthetic compound, 2-(3-hydroxyphenyl)-5-methylnaphthyridin-4-one (CSC-3436), on non-small cell lung cancer (NSCLC) cells.
Methods
Cell viability was determined by MTT assay. Cell cycle distribution was assessed by propidium iodide staining and subjected to flow cytometry analysis. Protein expression was detected by western blot analysis. Pharmacological inhibitors and shRNAs were applied to examine the possible pathways involved CSC-3436-inhibited viability of NSCLC cells.
Results
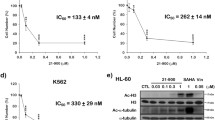
CSC-3436 decreased NSCLC cell viability by inducing apoptosis. In vivo and in vitro tubulin polymerization assays revealed that CSC-3463 caused tubulin depolymerization by directly binding to the colchicine-binding site. Furthermore, CSC-3436 caused the mitotic arrest with a marked activation of cyclin-dependent kinase 1 (CDK1) and increased the expression of phospho-Ser/Thr-Pro mitotic protein monoclonal 2. The CDK1 inhibitor, roscovitine, reversed the CSC-3436-induced upregulation of CDK1 activity as well as the mitotic arrest. DNA damage response kinases, including ataxia telangiectasia mutated (ATM), ATM and Rad3-related, DNA-dependent protein kinase, checkpoint kinase 1, and checkpoint kinase 2, were phosphorylated and activated by CSC-3436. c-Jun N-terminal kinase was activated by CSC-3436 and involved in the regulation of mitotic arrest and apoptosis. CSC-3436-induced apoptosis was accompanied by the activation of pro-apoptotic factors FADD, TRADD, and RIP and the inactivation of anti-apoptotic proteins Bcl-2 and Bcl-xL, resulting in the cleavage and subsequent activation of caspases.
Conclusions
Our results reveal the cellular events in which CSC-3436 induces tumor cell death and demonstrate that CSC-3436 is a potential tubulin-disrupting agent for antitumor therapy against NSCLC.






Similar content being viewed by others
Abbreviations
- ATM:
-
Ataxia telangiectasia mutated
- ATR:
-
ATM and Rad3-related
- CDK1:
-
Cyclin-dependent kinase 1
- Chk1:
-
Checkpoint kinase 1
- Chk2:
-
Checkpoint kinase 2
- CSC-3436:
-
2-(3-Hydroxyphenyl)-5-methylnaphthyridin-4-one
- DAPI:
-
4ʹ,6ʹ-Diamino-2-phenylindole
- DNA-PK:
-
DNA-dependent protein kinase
- FBS:
-
Fetal bovine serum
- FITC:
-
Fluorescein isothiocyanate
- JNK:
-
c-Jun N-terminal kinase
- MPM-2:
-
Phospho-Ser/Thr-Pro mitotic protein monoclonal 2
- NSCLC:
-
Non-small cell lung cancer
- PARP:
-
Poly(ADP-ribose)polymerase
References
Siegel RL, Miller KD, Jemal A (2015) Cancer statistics, 2015. CA Cancer J Clin 65:5–29
Herbst RS, Heymach JV, Lippman SM (2008) Lung cancer. N Engl J Med 359:1367–1380
Yang CH, Chen MC, Cheng AL, Hsu CH, Yeh KH, Yu YC, Whang-Peng J, Yang PC (2005) Survival outcome of inoperable non-small cell lung cancer patients receiving conventional dose epirubicin and Paclitaxel as first-line treatment. Oncology 68:350–355
Kuwabara K, Matsuda S, Fushimi K, Anan M, Ishikawa KB, Horiguchi H, Hayashida K, Fujimori K (2009) Differences in practice patterns and costs between small cell and non-small cell lung cancer patients in Japan. Tohoku J Exp Med 217:29–35
Wade RH (2009) On and around microtubules: an overview. Mol Biotechnol 43:177–191
Dumontet C, Jordan MA (2010) Microtubule-binding agents: a dynamic field of cancer therapeutics. Nat Rev Drug Discov 9:790–803
Johnson CE, Huang YY, Parrish AB, Smith MI, Vaughn AE, Zhang Q, Wright KM, Van Dyke T, Wechsler-Reya RJ, Kornbluth S, Ml Deshmukh (2007) Differential Apaf-1 levels allow cytochrome c to induce apoptosis in brain tumors but not in normal neural tissues. Proc Natl Acad Sci USA 104:20820–20825
Bhalla KN (2003) Microtubule-targeting anticancer agents and apoptosis. Oncogene 22:9075–9086
Esteve MA, Carre M, Braguer D (2007) Microtubules in apoptosis induction: are they necessary? Curr Cancer Drug Targets 7:713–729
Allan LA, Clarke PR (2007) Phosphorylation of caspase-9 by CDK1/cyclin B1 protects mitotic cells against apoptosis. Mol Cell 26:301–310
Lolli G, Johnson LN (2005) CAK-Cyclin-dependent Activating Kinase: a key kinase in cell cycle control and a target for drugs? Cell Cycle 4:572–577
Malumbres M, Barbacid M (2005) Mammalian cyclin-dependent kinases. Trends Biochem Sci 30:630–641
Castedo M, Perfettini JL, Roumier T, Kroemer G (2002) Cyclin-dependent kinase-1: linking apoptosis to cell cycle and mitotic catastrophe. Cell Death Differ 9:1287–1293
Ibrado AM, Kim CN, Bhalla K (1998) Temporal relationship of CDK1 activation and mitotic arrest to cytosolic accumulation of cytochrome c and caspase-3 activity during Taxol-induced apoptosis of human AML HL-60 cells. Leukemia 12:1930–1936
Sakurikar N, Eichhorn JM, Chambers TC (2012) Cyclin dependent kinase-1 (Cdk1)/cyclin B1 dictates cell fate after mitotic arrest via phosphorylation of regulation of anti-apoptotic Bcl-2 proteins. J Bio Chem 287:39193–39204
Manthey JA, Grohmann K, Guthrie N (2001) Biological properties of citrus flavonoids pertaining to cancer and inflammation. Curr Med Chem 8:135–153
Wang HK, Xia Y, Yang ZY, Natschke SL, Lee KH (1998) Recent advances in the discovery and development of flavonoids and their analogues as antitumor and anti-HIV agent. Adv Exp Med Biol 439:191–225
Tapia C, Kutzner H, Mentzel T, Savic S, Baumhoer D, Glatz K (2006) Two mitosis-specific antibodies, MPM-2 and phospho-histone H3 (Ser 28), allow rapid and precise determination of mitotic activity. Am J Surg Pathol 30:83–89
Elledge SJ (1996) Cell cycle checkpoints: preventing an identity crisis. Science 274:1664–1672
Vermeulen K, Berneman ZN, Van Bockstaele DR (2003) Cell cycle and apoptosis. Cell Prolif 36:165–175
Crasta K, Ganem NJ, Dagher R, Lantermann AB, Ivanova EV, Pan Y, Nezi L, Protopopov A, Chowdhury D, Pellman D (2012) DNA breaks and chromosome pulverization from errors in mitosis. Nature 482:53–58
Huang X, Traganos F, Darzynkiewicz Z (2003) DNA damage induced by DNA topoisomerase I- and topoisomerase II-inhibitors detected by histone H2AX phosphorylation in relation to the cell cycle phase and apoptosis. Cell Cycle 2:614–619
Furuta T, Takemura H, Liao ZY, Aune GJ, Redon C, Sedelinikova OA, Pilch DR, Rogakou EP, Celeste A, Chen HT, Nussenzweig A, Aladjem MI, Bonner WM, Pommier Y (2003) Phosphorylation of histone H2AX and activation of Mre11, Rad50, and Nbs1 in response to replication-dependent DNA double-strand breaks induced by mammalian DNA topoisomerase I cleavage complexes. J Biol Chem 278:20303–20312
Bacus SS, Gudkov AV, Lowe M, Lyass L, Yung Y, Komarov AP, Keyomarsi K, Yarden Y, Seger R (2001) Taxol-induced apoptosis depends on MAP kinase pathways (ERK and p38) and is independent of p53. Oncogene 20:147–155
Boldt S, Weidle UH, Kolch W (2002) The role of MAPK pathways in the action of chemotherapeutic drugs. Carcinogenesis 23:1831–1838
Selimovic D, Hassan M, Haikel Y, Hengge UR (2008) Taxol-induced mitochondrial stress in melanoma cells is mediated by activation of c-Jun N-terminal kinase (JNK) and p38 pathways via uncoupling protein 2. Cell Signal 20:311–322
Beardmore VA, Ahonen LJ, Gorbsky GJ, Kallio MJ (2004) Survivin dynamics increases at centromeres during G2/M phase transition and is regulated by microtubule-attachment and Aurora B kinase activity. J Cell Sci 117:4033–4042
Carmena M, Earnshaw WC (2003) The cellular geography f aurora kinases. Nat Rev Mol Cell Biol 4:842–854
Nitta M, Kobayashi O, Honda S, Hirota T, Kuninaka S, Marumoto T, Ushio Y, Saya H (2004) Spindle checkpoint function is required for mitotic catastrophe induced by DNA damaging agents. Oncogene 23:6548–6558
Ducommun B, Brambilla P, Felix MA, Franza BR Jr, Karsenti E, Draetta G (1991) Cdc2 phosphorylation is required for its interaction with cyclin. EMBO J 10:3311–3319
Terrano DT, Upreti M, Chambersb TC (2010) Cyclin-dependent kinase 1-mediated Bcl-xL/Bcl-2 phosphorylation acts as a functional link coupling mitotic arrest and apoptosis. Mol Cell Biol 30:640–656
Lu C, Zhu F, Cho YY, Tang F, Zykova T, Ma WY, Bode AM, Don Z (2006) Cell apoptosis: requirement of H2AX in DNA ladder formation, but not for the activation of caspase-3. Mol Cell 23:121–132
Liu J, Lin A (2005) Role of JNK activation in apoptosis: a double-edged sword. Cell Res 15:36–42
Tournier C, Hess P, Yang DD, Xu J, Turner TK, Nimnual A, Bar-Sagi D, Jones SN, Flavell RA, Davis RJ (2000) Requirement of JNK for stress-induced activation of the cytochrome c mediated death pathway. Science 288:870–874
Gutierrez GJ, Tsuji T, Cross JV, Davis RJ, Templeton DJ, Jiang W, Ronai ZA (2010) JNK-mediated phosphorylation of Cdc25C regulates cell cycle entry and G(2)/M DNA damage checkpoint. J Biol Chem 285:14217–14228
Chu R, Terrano DT, Chambers TC (2012) Cdk1/cyclin B plays a key role in mitotic arrest-induced apoptosis by phosphorylation of Mcl-1, promoting its degradation and freeing Bak from sequestration. Biochem Pharmacol 83:199–206
Danial NN, Korsmeyer SJ (2004) Cell death: critical control points. Cell 116:205–219
Jordan MA, Kamath K (2007) How do microtubule-targeted drugs work? An overview. Curr Cancer Drug Targets 7:730–742
Berthelet J, Dubrez L (2013) Regulation of apoptosis by inhibitors of apoptosis (IAPs). Cell 2:163–187
Mita AC, Mita MM, Nawrocki ST, Giles FJ (2008) Survivin: key regulator of mitosis and apoptosis and novel target for cancer therapeutics. Clin Cancer Res 14:5000–5005
Lens SM, Wolthuis RM, Klompmaker R et al (2003) Survivin is required for a sustained spindle checkpoint arrest in response to lack of tension. EMBO J 22:2934–2947
Shimada K, Matsuyoshi S, Nakamura M, Ishida E, Kishi M, Konishi N (2004) Phosphorylation of FADD is critical for sensitivity to anticancer drug-induced apoptosis. Carcinogenesis 25:1089–1097
Zhang X, Vallabhaneni R, Loughran PA, Shapiro R, Yin XM, Yuan Y, Billiar TR (2008) Changes in FADD levels, distribution, and phoshorylation in TNF alpha-induced apoptosis in hepatocytes is caspase-3, caspase-8 and BID dependent. Apoptosis 13:983–992
Acknowledgments
This work was supported by the National Science Council of the Republic of China (Grant Numbers NSC102-2325-B-039-005 and NSC102-2320-B-039-012 to S. C. Kuo, and NSC102-2321-B-039-004 to Y. L. Yu), the National Health Research Institute of the Republic of China (Grant Number NHRI-EX102-10245BI to Y. L. Yu), China Medical University Hospital (Grant Number DMR-104-097 and DMR-104-108 to L.-C. Chang), and CMU under the Aim for Top University Plan of the Ministry of Education, Taiwan.
Conflict of interest
No potential conflicts of interest were disclosed.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Ling-Chu Chang and Yung-Luen Yu have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chang, LC., Yu, YL., Liu, CY. et al. The newly synthesized 2-arylnaphthyridin-4-one, CSC-3436, induces apoptosis of non-small cell lung cancer cells by inhibiting tubulin dynamics and activating CDK1. Cancer Chemother Pharmacol 75, 1303–1315 (2015). https://doi.org/10.1007/s00280-015-2765-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-015-2765-0