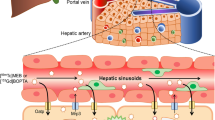
Abstract
Purpose
A recirculating isolated perfused rat liver model was used to investigate the hepatobiliary disposition of etoposide and the effects of cyclosporine A (CyA) on the pattern of drug disposition in the bile and uptake in the liver.
Methods
The portal vein, bile duct, and superior vena cava were cannulated in four groups of rats. The perfusions were conducted in the control group, which only received 10 µg/ml etoposide, and the tested groups which received etoposide and CyA in 0.4, 2, and 10 mg/kg doses. Perfusate and bile samples were collected up to 180 min.
Results
The determination of etoposide in the samples and homogenized liver by the high-performance liquid chromatography method showed that the administration of CyA led to significant changes in the hepatic excretion (E h), hepatic clearance (CL h), and half-life (T 1/2) of etoposide in the CyA 2 and 10 mg/kg treatment groups but not in 0.4 mg/kg group. The volume of the bile decreased to 64 and 45 % and biliary clearance (CL b) of etoposide reduced by 73 and 82 % in 0.4 and 2 mg/kg CyA group, respectively, when compared with the control group.
Conclusions
These results demonstrated the dose-dependant non-specific inhibitory effects of CyA on p-glycoproteins, multidrug resistance protein 2, bile salt export pump, and organic anion-transporting polypeptide, the drug transporters responsible for etoposide hepatobiliary disposition, hepatic uptake, and bile formation in rat.





Similar content being viewed by others
References
Faber KN, Muller M, Jansen PL (2003) Drug transporter proteins in the liver. Adv Drug Deliv Rev 55:107–124. doi:10.1016/S0169-409X(02)00173-4
Kusuhara H, Sugiyama Y (2002) Role of transporters in the tissue-selective distribution and elimination of drugs: transporters in the liver, small intestine, brain and kidney. J Control Release 78:43–54. doi:10.1016/S0168-3659(01)00480-1
Gillet JP, Efferth T, Remacle J (2007) Chemotherapy-induced resistance by ATP-binding cassette transporter genes. Biochem Biophys 1775:237–262. doi:10.1016/j.bbcan.2007.05.002
Schinkel AH, Jonker JW (2003) Mammalian drug efflux transporters of the ATP binding cassette (ABC) family: an overview. Adv Drug Deliv Rev 55:3–29. doi:10.1016/S0169-409X(02)00169-2
Clark PI, Slevin ML (1987) The clinical pharmacology of etoposide and teniposide. Clin Pharmacokinet 12:223–252. doi:10.2165/00003088-198712040-00001
Slevin ML (1991) The clinical pharmacology of etoposide. Cancer 67:319–329. doi:10.1002/1097-0142(19910101)67:1+<319:AID-CNCR2820671319>3.0.CO;2-D
Jurien LS, Fan L, Wagenaar E, Vlaming ML, van Tellingen O, Beijnen JH, Schinkel AH (2010) P-glycoprotein, Abbc2 and Abcc3 determine the pharmacokinetic of etoposide. Clin Cancer Res 16:130–140. doi:10.1158/1078-0432.CCR-09-1321
Allen JD, van Dort SC, Buitelaar M, van Tellingen O, Schinkel AH (2003) Mouse breast cancer resistance protein (Bcrp1/Abcg2) mediated etoposide resistance and transport, but etoposide oral availability is limited primarily by p-glycoprotein. Cancer Res 63:1339–1344
Lacayo NJ, Lum BL, Becton DL, Weinstein H, Ravindranath Y, Chang MN et al (2002) Pharmacokinetic interaction of cyclosporine with etoposide and mitoxantrone in children with acute myeloid leukaemia. Lukemia 16:920–927. doi:10.1038/sj/leu/2402455
Bisogno G, Cowie F, Boddy A, Thomas HD, Dick G, Pinkerton CR (1997) High dose cyclosporine with etoposide toxicity and pharmacokinetic interaction in children with solid tumours. Br J Cancer 77:2304–2309. doi:10.1038/bjc.1998.383
Hnadler JA, Kossor DC, Goldstein RS (1994) Assessment of hepatobiliary functions in vivo and ex vivo in the rat. J Pharmacol Toxicol Methods 31:11–19. doi:10.1016/1056-8719(94)90024-8
Saadati R, Dadashzadeh S (2011) Simple and efficient HPLC-UV quantitation of etoposide and its cis-isomer in rat micro-volume plasma and tissue sample: application to pharmacokinetic and biodistribution studies. J Liq Chromatogr Relat Technol 34:2130–2148. doi:10.1080/10826076.2011.585483
Yahanda AM, Alder KM, Fisher GA, Brophy NA, Halsey J, Hardy RI, Gosland MP, Lum BL, Sikic BI (1992) Phase I trial of etoposide with cyclosporine as a modulator of multidrug resistance. J Clin Oncol 10:1624–1634
Lum BL, Kaubisch S, Yahanda AM, Adler KM, Jew L, Ehsan MN, Brophy NA, Halsey J, Gosland MP, Sikic BI (1992) Alteration of etoposide pharmacokinetics and pharmacodynamics by cyclosporine in a phase I trial to modulate multidrug resistance. J Clin Oncol 10:1635–1642
Burgio DE, Gosland MP, McNamara PJ (1998) Effects of P-glycoprotein modulators on etoposide elimination and central nervous system distribution. J Pharmacol Exp Ther 287:911–917
Rouini MR, Ghazi-Khansari M, Ardakani YH, Dasian Z, Lavasani H (2008) A disposition kinetic study of tramadol in rat perfused liver. Biopharm Drug Dispos 29:231–235. doi:10.1002/bdd.606
Mehvar R, Chimalakonda AP (2004) Hepatic disposition of cyclosporine A in isolated perfused rat livers. J Pharm Pharm Sci 7:47–54
Parasrampuria R, Mehvar R (2010) Effect of P-glycoprotein and MRP2 inhibitors on the hepatobiliary disposition of rhodamine 123 and its glucuronidated metabolite in isolated perfused rat livers. J Pharm Sci 99:455–466. doi:10.1002/jps.21831
Parasrampuria R, Mehvar R (2010) Dose-dependent inhibition of transporter-mediated hepatic uptake and biliary excretion of methotrexate by cyclosporine A in an isolated perfused rat liver model. J Pharm Sci 99:5060–5069. doi:10.1002/jps.22187
Yamazaki M, Li B, Louie SW, Pudvah NT, Stocco R, Wongb W et al (2005) Effect of fibrate on human organic anion-transporting polypeptide 1B1, multidrug resistance protein 2 and P-glycoprotein mediated transport. Xenobiotica 35:737–753. doi:10.1080/00498250500136676
Zelcer N, Saeki T, Reid G, Beijnen JH, Borst P (2001) Characterization of drug transport by the human multidrug resistance protein 3(ABCC3). J Biol Chem 276:46400–46407. doi:10.1074/jbc.M107041200
Kool M, van der Linden M, de Haas M, Scheffer GL, de Vree JM, Smith AJ et al (1999) MRP3 an organic anion transporter able to transport anti-cancer drug. Proc Natl Acad Sci USA 96:6914–6919
Balley DG (2010) Fruit juice inhibition of uptake transport: a new type of food-drug interaction. Br J Clin Pharmacol 70:645–655. doi:10.1073/pnas.96.12.6914
Bohme M, Buchler M, Muller M, Keppler D (1993) Differential inhibition by cyclosporins of primary-active ATP-dependent transporters in the hepatocyte canalicular membrane. FEBS Lett 333:193–196. doi:10.1016/0014-5793(93)80403-H
Shitar Y, Nagamatsu Y, Wada S, Sugiyama Y, Horie T (2009) Long lasting inhibition of transporter mediated hepatic uptake of sulfobromophthalein by cyclosporine A in rats. Drug Metab Dispos 37:1172–1178. doi:10.1124/dmd.108.025544
Acknowledgments
This study was part of a bio-pharmaceutical and pharmacological thesis supported by Tehran University of Medical Sciences.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the Animal Ethic Committee and Institutional Review Board of Pharmaceutical Research Centre of Tehran University of Medical Sciences.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khezrian, M., Sheikholeslami, B., Dadashzadeh, S. et al. Effects of cyclosporine A on the hepatobiliary disposition and hepatic uptake of etoposide in an isolated perfused rat liver model. Cancer Chemother Pharmacol 75, 961–968 (2015). https://doi.org/10.1007/s00280-015-2719-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-015-2719-6