Abstract
Purpose
MK-0888 is an investigational VEGFR-2 inhibitor with demonstrated potent in vitro enzyme activity. Clinical investigation in healthy volunteers and cancer patients was undertaken to evaluate its pharmacokinetic properties and early safety profile. Early data were used to guide whether further clinical development was warranted.
Methods
Five phase I studies were conducted. Studies 1–4 were conducted in healthy male volunteers and examined safety and pharmacokinetics across a dose range of 0.5–100 mg. Single-dose and limited multiple-dose escalations were performed. Three formulations and food effect were assessed. Study 5 was a dose escalation study in cancer patients, evaluating pharmacokinetics and safety at doses of 6–100 mg administered up to twice daily.
Results
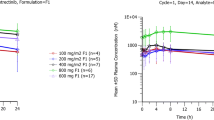
Safety: MK-0888 was generally well tolerated in healthy volunteers at single doses up to 100 mg and in cancer patients at doses up to 100 mg twice daily. Pharmacokinetics: After single-dose administration, MK-0888 was readily absorbed with a T max of 4–5 h and a half-life of 11.3–22.7 h. AUC, C max, and C 24h increased in a slightly less than dose proportional manner. With longer duration multiple-dose administration (2 weeks), trough concentrations decreased from Day 2 at doses of 50 mg twice daily and higher, suggestive of autoinduction of metabolism. The efficacious trough pharmacokinetic target was not attained at steady state.
Conclusions
The pharmacokinetic behavior of MK-0888 does not support continued development. The early pharmacokinetic profile of the compound provides important information as to the probability of success of MK-0888 achieving efficacious exposures.

Similar content being viewed by others
References
Bruce D, Tan PH (2011) Vascular endothelial growth factor receptors and the therapeutic targeting of angiogenesis in cancer: where do we go from here? Cell Commun Adhes 18:85–103. doi:10.3109/15419061.2011.619673
Hicklin DJ, Ellis LM (2005) Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol 23:1011–1027. doi:10.1200/JCO.2005.06.081
Simon R, Rubinstein L, Arbuck SG, Christian MC (1997) Accelerated titration designs for phase I clinical trials in oncology. J Natl Cancer Inst 89:1138–1147. doi:10.1093/jnci/89.15.1138
Iwamoto M, Iannone R, Wagner JA (2012) Use of healthy volunteers drives clinical oncology drug development decision making. Clin Pharmacol Ther 92:571–574. doi:10.1038/clpt.2012.157
Acknowledgments
Deepest appreciation and thanks go to all of the subjects and patients who participated in the clinical studies referenced above. Additional acknowledgement for scientific support: Manuel Modiano, Malgorzata Wojtowicz, Eric Rubin, Bart Keymeulin, Howard Greenberg, Steven Ramael, Lucas Van Bortel, Kenneth Lasseter, Stephen C. Beck, Mary Flynn, Aimee Dallob, Chau Thach, Lihong Du, Yang Xu, Yih Lee, Inge de Lepeleire, Tine Latham, Sarah Liou, Kathryn Mazina, and Paul Deutsch.
Conflict of interest
All authors are or were employees of Merck Sharp & Dohme Corp.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
280_2014_2649_MOESM3_ESM.pdf
Supplementary material 3 Figure S2. Individual and mean MK-0888 AUC0-∞ values in young, healthy, male subjects following single oral doses of MK-0888 administered in the fasted state (Studies 1, 2, and 4) (PDF 42 kb)
Rights and permissions
About this article
Cite this article
Iwamoto, M., Friedman, E.J., Sepp-Lorenzino, L. et al. Pharmacokinetic/pharmacodynamic-based decision making in the development of MK-0888, a VEGFR-2/FLT-3 kinase inhibitor. Cancer Chemother Pharmacol 75, 333–342 (2015). https://doi.org/10.1007/s00280-014-2649-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-014-2649-8