Abstract
Purpose
To date, gemcitabine-based or fluoropyrimidine-based regimens are recommended for unresectable advanced biliary tract cancer. Then, we conducted a phase I study of gemcitabine/cisplatin and S-1 that is an oral fluoropyrimidine. The aim of this study was to determine the dose-limiting toxicity (DLT), maximum-tolerated dose, and a recommended phase II dose of S-1. Response was assessed as a secondary endpoint.
Patients and methods
Patients who have been diagnosed with unresectable or postoperative recurrent biliary tract cancer received cisplatin (25 mg/m2 i.v. for 120 min) followed by gemcitabine (1,000 mg/m2 i.v. for 30 min) on days 1 and 8, and oral S-1 on alternate days; this regimen was repeated at 21-day intervals. A standard ‘3 + 3’ phase I dose-escalation design was adopted. This study was registered with University hospital Medical Information Network (UMIN) Center in Japan, number UMIN000008415.
Results
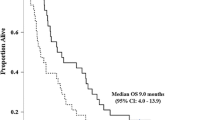
Twelve patients were evaluable in this study. No patients developed DLTs. Recommended dose of S-1 was 80 (<1.25 m2), 100 (1.25 ≤ 1.5 m2), and 120 mg (1.5 m2≥) per day. One patient could achieve conversion to curative surgery.
Conclusion
This phase I study was performed safely and demonstrated encouraging response.


Similar content being viewed by others
References
Ministry of Health, Labour and Welfare in Japan. http://www.mhlw.go.jp/seisaku/24.html
Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM (2010) Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 127:2893–2917
de Groen PC, Gores GJ, LaRusso NF, Gunderson LL, Nagorney DM (1999) Biliary tract cancers. N Engl J Med 341:1368–1378
Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira SP, Roughton M, Bridgewater J (2010) ABC-02 Trial Investigators. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 362:1273–1281
Sasaki T, Isayama H, Nakai Y, Mizuno S, Yamamoto K, Yagioka H, Yashima Y, Kawakubo K, Kogure H, Togawa O, Matsubara S, Ito Y, Sasahira N, Hirano K, Tsujino T, Toda N, Tada M, Omata M, Koike K (2012) Multicenter phase II study of S-1 monotherapy as second-line chemotherapy for advanced biliary tract cancer refractory to gemcitabine. Invest New Drugs 30:708–713
Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama W, Toh Y, Nagaie T, Takagi S, Yamamura Y, Yanaoka K, Orita H, Takeuchi M (2008) S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 9:215–221
Kogashiwa Y, Nagafuji H, Kohno N (2012) Feasibility of concurrent chemoradiotherapy with S-1 administered on alternate days for elderly patients with head and neck cancer. Anticancer Res 32:4035–4040
Tatebe S, Tsujitani S, Nakamura S, Shimizu T, Yamane N, Nishidoi H, Kurisu Y, Kanayama H, Ogawa H, Ikeguchi M (2014) Feasibility study of alternate-day S-1 as adjuvant chemotherapy for gastric cancer: a randomized controlled trial. Gastric Cancer 17:508–513
Kang MJ, Lee JL, Kim TW, Lee SS, Ahn S, Park do H, Lee SS, Seo DW, Lee SK, Kim MH (2012) Randomized phase II trial of S-1 and cisplatin versus gemcitabine and cisplatin in patients with advanced biliary tract adenocarcinoma. Acta Oncol 51:860–866
Sasaki T, Isayama H, Nakai Y, Ito Y, Yasuda I, Toda N, Kogure H, Hanada K, Maguchi H, Sasahira N, Kamada H, Mukai T, Okabe Y, Hasebe O, Maetani I, Koike K (2013) A randomized phase II study of gemcitabine and S-1 combination therapy versus gemcitabine monotherapy for advanced biliary tract cancer. Cancer Chemother Pharmacol 71:973–979
Morizane C, Okusaka T, Mizusawa J, Takashima A, Ueno M, Ikeda M, Hamamoto Y, Ishii H, Boku N, Furuse J (2013) Randomized phase II study of gemcitabine plus S-1 versus S-1 in advanced biliary tract cancer: a Japan Clinical Oncology Group trial (JCOG 0805). Cancer Sci 104:1211–1216
Santini D, Virzi V, Vasile E, Vincenzi B, Catalano V, Graziano F, Masi G, Bronte G, Russo A, Falcone A, Tonini G (2012) A phase II trial of fixed-dose rate gemcitabine plus capecitabine in metastatic/advanced biliary tract cancer patients. Oncology 82:75–82
Borbath I, Ceratti A, Verslype C, Demols A, Delaunoit T, Laurent S, Deleporte A, Vergauwe P, Van Maanen A, Sempoux C, Van Cutsem E, Van Laethem JL (2013) Belgian Group of Digestive Oncology. Combination of gemcitabine and cetuximab in patients with advanced cholangiocarcinoma: a phase II study of the Belgian Group of Digestive Oncology. Ann Oncol 24:2824–2829
Sohal DP, Mykulowycz K, Uehara T, Teitelbaum UR, Damjanov N, Giantonio BJ, Carberry M, Wissel P, Jacobs-Small M, O’Dwyer PJ, Sepulveda A, Sun W (2013) A phase II trial of gemcitabine, irinotecan and panitumumab in advanced cholangiocarcinoma. Ann Oncol 24:3061–3065
Hezel AF, Noel MS, Allen JN, Abrams TA, Yurgelun M, Faris JE, Goyal L, Clark JW, Blaszkowsky LS, Murphy JE, Zheng H, Khorana AA, Connolly GC, Hyrien O, Baran A, Herr M, Ng K, Sheehan S, Harris DJ, Regan E, Borger DR, Iafrate AJ, Fuchs C, Ryan DP, Zhu AX (2014). Phase II study of gemcitabine, oxaliplatin in combination with panitumumab in KRAS wild-type unresectable or metastatic biliary tract and gallbladder cancer. Br J Cancer (in press)
Kanai M, Hatano E, Kobayashi S, Fujiwara Y, Sakai D, Kodama Y, Ajiki T, Nagano H, Ioka T (2012) Phase I trial of oral S-1 combined with gemcitabine and cisplatin for advanced biliary tract cancer (KHBO1002). Cancer Chemother Pharmacol 69:1181–1188
Acknowledgments
The authors thank all patients who consented to participate in this study and nurses who took care of patients at the outpatient section of our departments.
Conflict of interest
The authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Uwagawa, T., Sakamoto, T., Abe, K. et al. Phase I trial of S-1 every other day in combination with gemcitabine/cisplatin for inoperable biliary tract cancer. Cancer Chemother Pharmacol 75, 191–196 (2015). https://doi.org/10.1007/s00280-014-2636-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-014-2636-0