Abstract
Purpose
To investigate the efficacy of gemcitabine plus uracil–tegafur (UFT) combination chemotherapy as a salvage treatment in patients with metastatic colorectal cancer (MCRC).
Methods
This single-arm phase II study was conducted at three institutions in Korea. Patients with MCRC refractory to fluoropyrimidine, oxaliplatin and irinotecan were enrolled. Gemcitabine 800 mg/m2 was administered intravenously on days 1, 8 and 15. UFT 200 mg/m2/day was taken orally in three divided doses on days 1–21. Cycles were repeated every 4 weeks, and tumor evaluation was carried out every 8 weeks. The primary endpoint of this study was 8-week progression-free survival (PFS) rate.
Results
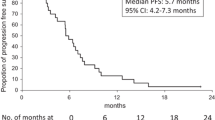
Forty-one patients were enrolled. Fourteen patients received gemcitabine/UFT as a third-line treatment and 37 patients as a fourth-line or later-line therapy. Toxicities were easily manageable, and non-hematologic toxicities of ≥grade 3 were rare. The most common toxicity of ≥grade 3 was neutropenia (20.0 %). One patient showed partial response (response rate, 2.4 %) and 14 (34.1 %) showed stable disease. The 8-week PFS rate was 42.3 %. The median PFS was 1.7 months [95 % confidence interval (CI) 1.6–1.8 months], and the median overall survival was 9.2 months (95 % CI 5.8–12.6 months).
Conclusions
Overall efficacy of gemcitabine/UFT in refractory MCRC was unsatisfactory. However, we could find a minor proportion of patients who showed prolonged tumor stabilization to gemcitabine/UFT. Further studies are warranted to identify a patient subgroup that might have benefits from gemcitabine/UFT therapy.

Similar content being viewed by others
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61:69–90
Jung KW, Won YJ, Kong HJ, Oh CM, Seo HG, Lee JS (2013) Prediction of cancer incidence and mortality in Korea, 2013. Cancer Res Treat 45:15–21
Cancer M-AGi (1998) Efficacy of intravenous continuous infusion of fluorouracil compared with bolus administration in advanced colorectal cancer. Meta-analysis group in cancer. J Clin Oncol 16:301–308
Van Cutsem E, Twelves C, Cassidy J, Allman D, Bajetta E, Boyer M, Bugat R, Findlay M, Frings S, Jahn M, McKendrick J, Osterwalder B, Perez-Manga G, Rosso R, Rougier P, Schmiegel WH, Seitz JF, Thompson P, Vieitez JM, Weitzel C, Harper P (2001) Oral capecitabine compared with intravenous fluorouracil plus leucovorin in patients with metastatic colorectal cancer: results of a large phase III study. J Clin Oncol 19:4097–4106
Hoff PM, Ansari R, Batist G, Cox J, Kocha W, Kuperminc M, Maroun J, Walde D, Weaver C, Harrison E, Burger HU, Osterwalder B, Wong AO, Wong R (2001) Comparison of oral capecitabine versus intravenous fluorouracil plus leucovorin as first-line treatment in 605 patients with metastatic colorectal cancer: results of a randomized phase III study. J Clin Oncol 19:2282–2292
Grothey A, Sargent D, Goldberg RM, Schmoll HJ (2004) Survival of patients with advanced colorectal cancer improves with the availability of fluorouracil–leucovorin, irinotecan, and oxaliplatin in the course of treatment. J Clin Oncol 22:1209–1214
Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F (2004) Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350:2335–2342
Van Cutsem E, Kohne CH, Lang I, Folprecht G, Nowacki MP, Cascinu S, Shchepotin I, Maurel J, Cunningham D, Tejpar S, Schlichting M, Zubel A, Celik I, Rougier P, Ciardiello F (2011) Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol 29:2011–2019
Bitossi R, Sculli CM, Tampellini M, Alabiso I, Brizzi MP, Ferrero A, Ottone A, Bellini E, Gorzegno G, Berruti A, Dogliotti L (2008) Gemcitabine and protracted 5-fluorouracil infusion as third-line chemotherapy in refractory colorectal cancer patients. Anticancer Res 28:3055–3060
Iqbal S, Yang D, Cole S, El-Khoueiry AB, Boswell W, Agafitei R, Lujan R, Lenz HJ (2009) Phase II study of capecitabine and gemcitabine in patients with metastatic colorectal cancer (mCRC). J Clin Oncol (Meeting Abstracts) 27: (suppl; abstr e15077)
Salgado M, Reboredo M, Mendez JC, Quintero G, Pellon ML, Romero C, Jorge M, Montes AF, Valladares-Ayerbes M, Ramos M, Varela S, Alonso MA (2013) Gemcitabine and capecitabine as third- or later-line therapy for refractory advanced colorectal cancer: a retrospective study. Anticancer Res 33:4089–4096
Moore DF Jr, Pazdur R, Daugherty K, Tarassoff P, Abbruzzese JL (1992) Phase II study of gemcitabine in advanced colorectal adenocarcinoma. Invest New Drugs 10:323–325
Mani S, Kugler JW, Knost JA, Sciortino DF, Gibbons J, Garcia JC, Ansari RH, Schilsky RL, Vokes EE (1998) Phase II trial of 150-minute weekly infusion of gemcitabine in advanced colorectal cancer: minimal activity in colorectal cancer. Invest New Drugs 16:275–278
Merl M, Hoimes C, Pham T, Saif MW (2009) Is there a palliative benefit of gemcitabine plus fluoropyrimidines in patients with refractory colorectal cancer? A review of the literature previously presented: poster at the 2008 Gastrointestinal Cancer Symposium (Abstract No. 512). Expert Opin Investig Drugs 18:1257–1264
Pachon V, Garcia-Alfonso P, Iglesias L, Siso I, Abad G, Khosravi P, Diaz V, Perez-Manga G (2005) Gemcitabine plus continuous infusion of 5-FU for heavily pretreated advanced colorectal cancer patients. Phase I/II study. J Clin Oncol (Meeting Abstracts) 23: abstract 3735
Qiu M, Bi F, Liu J, Li Q, Yi C (2011) Gemcitabine and capecitabine as third-line treatment in patients with metastatic colorectal cancer after failure of irinotecan and oxaliplatin. J Clin Oncol (Meeting Abstracts) 29: (suppl 4; abstr 620)
Saif MW, Kaley K, Penney R, Hotchkiss S, Syrigos KN, Strimpakos AS (2011) The efficacy of gemcitabine as salvage treatment in patients with refractory advanced colorectal cancer (CRC): a single institution experience. Anticancer Res 31:2971–2974
Hernandez M, Jiménez P, Garcia LF, Alvarez C, Pérez Q, Rodriguez DJ, Ruiz AL, Sánchez L, Torres W, Esther Uriol E, Menendez MD, Prado J (2013) Biweekly gemcitabine and capecitabine in heavily pretreated patients with metastatic colorectal cancer (mCRC). J Clin Oncol (Meeting Abstracts) 31: (suppl; abstr e14617)
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
Van Cutsem E, Peeters M, Siena S, Humblet Y, Hendlisz A, Neyns B, Canon JL, Van Laethem JL, Maurel J, Richardson G, Wolf M, Amado RG (2007) Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol 25:1658–1664
Karapetis CS, Khambata-Ford S, Jonker DJ, O’Callaghan CJ, Tu D, Tebbutt NC, Simes RJ, Chalchal H, Shapiro JD, Robitaille S, Price TJ, Shepherd L, Au HJ, Langer C, Moore MJ, Zalcberg JR (2008) K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med 359:1757–1765
Amado RG, Wolf M, Peeters M, Van Cutsem E, Siena S, Freeman DJ, Juan T, Sikorski R, Suggs S, Radinsky R, Patterson SD, Chang DD (2008) Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol 26:1626–1634
Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, Humblet Y, Bouche O, Mineur L, Barone C, Adenis A, Tabernero J, Yoshino T, Lenz HJ, Goldberg RM, Sargent DJ, Cihon F, Cupit L, Wagner A, Laurent D (2013) Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 381:303–312
Madajewicz S, Waterhouse DM, Ritch PS, Khan MQ, Higby DJ, Leichman CG, Malik SK, Hentschel P, Gill JF, Zhao L, Nicol SJ (2012) Multicenter, randomized phase II trial of bevacizumab plus folinic acid, fluorouracil, gemcitabine (FFG) versus bevacizumab plus folinic acid, fluorouracil, oxaliplatin (FOLFOX4) as first-line therapy for patients with advanced colorectal cancer. Invest New Drugs 30:772–778
Bennouna J, Saunders M, Douillard JY (2009) The role of UFT in metastatic colorectal cancer. Oncology 76:301–310
Lembersky BC, Wieand HS, Petrelli NJ, O’Connell MJ, Colangelo LH, Smith RE, Seay TE, Giguere JK, Marshall ME, Jacobs AD, Colman LK, Soran A, Yothers G, Wolmark N (2006) Oral uracil and tegafur plus leucovorin compared with intravenous fluorouracil and leucovorin in stage II and III carcinoma of the colon: results from National Surgical Adjuvant Breast and Bowel Project Protocol C-06. J Clin Oncol 24:2059–2064
Kim TW, Kang HJ, Ahn JH, Lee K, Chang HM, Kang YK, Lee JS (2002) Phase II study of gemcitabine, UFT and leucovorin in patients with advanced pancreatic cancer. Acta Oncol 41:689–694
Sym SJ, Hong J, Ahn HJ, Park J, Cho EK, Lee JH, Lee WS, Baek JH, Park YH, Shin DB (2013) A phase II trial of salvage treatment with gemcitabine and S-1 combination in heavily pretreated patients with metastatic colorectal cancer. J Clin Oncol (Meeting Abstracts) 31: (suppl; abstr 3595)
Acknowledgments
This study was supported in part by grants from Yuhan Cooporation (Seoul, Korea). Gemcitabine and UFT were provided by Yuhan Corporation and Jeil Pharmaceutical (Seoul, Korea), respectively. We thank Medical Research Collaborating Center at Seoul National University Hospital for statistical assistance. We are also grateful to Yong Min Shin and Eun-Kyung Kim (Seoul National University Bundang Hospital) for their supports as clinical research coordinators.
Conflict of interest
We declare that we have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, KW., Kim, Y.J., Lee, KH. et al. Phase II trial of gemcitabine plus UFT as salvage treatment in oxaliplatin, irinotecan and fluoropyrimidine-refractory metastatic colorectal cancer. Cancer Chemother Pharmacol 74, 447–455 (2014). https://doi.org/10.1007/s00280-014-2515-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-014-2515-8