Abstract
Diffuse large B-cell lymphoma (DLBCL), with approximately 150,000 new cases worldwide each year, represent nearly 30% of all cases of non-Hodgkin lymphoma (NHL) and are phenotypically and genetically heterogeneous. A gene-expression profile (GEP) has identified at least three major subtypes of DLBCL, each of which has distinct clinical, biological, and genetic features: activated B-cell (ABC)-like DLBCL, germinal-center B-cell (GCB)-like DLBCL, and unclassified. Different origins are associated with different responses to chemotherapy and targeted agents. Despite DLBCL being a highly heterogeneous disease, more than 60% of patients with DLBCL can be cured after using rituximab combined with cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) to inhibit the growth of cancer cells while targeting the CD20 receptor. In recent decades, the improvement of diagnostic levels has led to a refinement classification of DLBCL and the development of new therapeutic approaches. The objective of this review was to summarize the latest studies examining genetic lesions and therapies for DLBCL.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Approximately 60% of patients with diffuse large B-cell lymphoma (DLBCL), the most common lymphoid malignancy in adults, can be cured with anti-CD20 antibody in combination with cyclophosphamide, doxorubicin, vincristine and prednisone (R-CHOP) [1]. The past few decades have seen numerous targeted therapies discovered, but many patients relapse or die due to their complications. In approximately one-third of patients treated with standard R-CHOP regimens, DLBCL remains the most challenging clinical problem [2, 3]. Due to the heterogeneity of this disease, the treatment effect is limited. In recent years, modern genome-wide molecular analysis of DLBCL has revealed multiple altered pathways associated with tumor development and metastasis, including responses to chemotherapy. Understanding the heterogeneity of this disease will be helpful to further improve treatment outcomes. With these methods, diagnostics and prognostic markers will be developed that are more accurate and reliable, providing opportunities for the development of precision medicine strategies aimed at addressing oncogenic addictions specific to each subtype of lymphoma. Here we summarize the latest data and discuss the genetics and therapies of DLBCL and the new agents in the frontline treatment of DLBCL.
Subtypes of DLBCL
The World Health Organization (WHO) has updated its classification of haematopoietic and lymphoid tissues for the 5th edition, a B-cell lymphoid proliferations and lymphomas (Table 1) [4]. Most DLBCLs arise de novo, but they can also originate from indolent lymphomas, such as follicular lymphoma (FL) [5,6,7,8], chronic lymphocytic leukemia (CLL), or small lymphocytic lymphoma (SLL) [9, 10]. As a secondary disease, DLBCL can also occur in patients who have received solid organ transplants or who are suffering from human immunodeficiency virus (HIV) [11,12,13].
Gene expression profiling
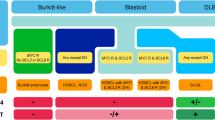
DLBCL can be divided into two main subgroups based on its cell-of-origin (COO): germinal center B-cells (GCBs) and non-GCBs. Different subgroups represent different molecular characteristics and clinical behavior [14]. Based on transcriptome sequencing, researchers found that there were different gene mutations among different subtypes of DLBCL [15,16,17]. The analyses have been based on COO analysis, immunohistochemistry (IHC) algorithms, and gene expression profiling (GEP) techniques, all indicating that DLBCL patients have a more common non-GCB phenotype, accounting for 59% to 75% of cases compared to 50% in patients with advanced-stage disease [18,19,20,21,22]. ABC subgroup patients with MYD88, CD79B, and NOTCH1 mutations have a poorer prognosis than patients with other mutations [16]. DLBCL subgroups with EZH2 mutations and BCL-2 translocations are associated with worse outcomes in GCB-DLBCL patients [16]. Similarly, double-hit/triple-hit (DH/TH) (~ 7%) is a type of high-grade B-cell lymphoma (HGBL), that has MYC, BCL-2 (~ 20%) or/and BCL-6(~ 14%) rearrangements [18, 22,23,24]. Due to the clear genetic and biological differences between ABC and GCB DLBCL, patients with ABC have a worse prognosis than those with GCB when treated with R-CHOP as a first-line treatment [25,26,27,28,29,30,31]. The treatments and outcomes for DLBCL subtypes see Table 2.
The use of microarrays to analyze gene expression profiles is another method to classify DLBCL in relation to different aspects of the disease’s biology. Tumor microenvironments (TMEs) are characterized by the differential expression of genes involved in oxidative phosphorylation and B-cell receptor (BCR) signaling as well as Molecular heterogeneity in diffuse large B-cell lymphoma and its implications in clinical diagnosis and treatment the inflammatory response of the host [34]. The pathogenesis of DLBCL involves somatic mutations that include chromosomal aberrations, translocations, and copy number changes in specific chromosomal regions. By RNA sequencing, a somatic mutation frequency of 3–6 mutations is observed, which is more common than renal cell carcinoma and acute leukemia (AL) but much less than solid tumors, such as melanomas or lung cancers (> 10 mutations) [35,36,37]. Each lymphoma has 20 to 400 different gene mutations that affect the coding DNA sequences [37,38,39]. Different gene mutations exist in different subtypes of DLBCL, which are usually related to the prognosis of patients, and some mutations only occur in specific subtypes (Fig. 1).
As gene next-generation sequencing is conducted more frequently in clinical examinations, the subgroups defined at the genetic level largely direct prognosis and therapeutic regimens. Subgroups based on genetics, although partially coincident with COO subgroups, show more accuracy. The BN2 subgroups contain 41% ABC and 19% GCB types as reported by Roland Schmitz and feature damage to NOTCH pathways; thus, BTK inhibitors can be used, especially ibrutinib [16]. Additionally, in the MCD, BN2 and EZB subgroups, especially PI3K signaling inhibitors can make results. Furthermore, the genetic subtypes indicate prognosis. Schmitz R and his workmates used the genetic algorithm, which did not use clinical information found that the four subtypes differed significantly in progression-free survival, the 5-year survival rates in the MCD, N1, BN2 and EZB subgroups are 26%, 36%, 65% and 68%, respectively [16]. G. W. Wright proposed extra ST2 and A53 subgroups, and discriminated EZB subgroups by whether MYC expressed as the significantly different survival results occurring [17]. A study that recruited 105 patients whose pathological gene sequencing data were available showed poor prognosis in the N1 and A53 subgroups. This more accurate classification can bring more value to both therapy and prognosis.
ABC subtype lesions
B-cell differentiation
One of the main mechanisms underlying the pathogenesis of DLBCL is the normal process of GCB during the development or occurrence of gene mutations. Deregulating BCL-6, the main regulator of GCB differentiation, directly affects this process. Various cellular functions are regulated by BCL-6, including DNA damage responses, cell cycle progression, and signal transduction [29, 40,41,42,43,44,45,46]. Chromosomal translocations affect BCL-6 (3q27) more frequently in ABC DLBCL than in GCB DLBCL, resulting in the deregulation of BCL-6 expression. BCL-6 expression is considered to be related to improved outcomes, reflecting the prognosis of GCB [47,48,49,50]. Some researchers have found that BCL-6 translocations affect the prognosis of patients, but relevant studies have not been fully confirmed [51,52,53].
The deletions of DLBCL often occur in 6q23 and 6q21 [54,55,56,57]. PRDM1 (PR domain containing 1, with ZNF domain) is a transcriptional repressor important for the terminal differentiation of B-cells into plasma cells. Alterations in BLIMP1 only occur in ABC subgroups. In addition, BCL-6 is one of the most important repressors of BLIMP1, and changes in BCL-6 can also affect BLIMP1. These findings indicate that translocations or mutations contribute to the development of ABC subgroups [57]. Chromosomal translocations and genomic gains in ABC subgroups target SPIB (19q13.3-q13.4) [58, 59]. BLIMP also targets SPIB, with high expression in ABC subgroups compared to GCB subgroups [60].
BCR and NF-κB signaling
Most changes in the ABC subgroup are caused by activation of the NF-κB signaling pathway. Genetic lesions among the different genes activate the NF-κB pathway, illustrating the many pathways that cause NF-κB activation in normal GCBs. Somatic mutations and deletions inactivate a relatively small number of genes, including TNFAIP3 (∼30%), MYD88 (∼30%), CARD11 (∼10%), TRAF5 (∼5%), CD79B, CD79A (∼20%) and TRAF2 (∼3%), while RANK (∼8%) is activated largely because of somatic mutations [37, 38, 61,62,63,64]. Overall, 20%-30% of DLBCLs have TNFAIP3 mutations, especially in ABC subgroups. Lymphoma cells inactivate TNFAIP3 and also negatively regulate NF-κB [49, 65, 66]. MYD88 mutations are present in approximately 30% of cases of ABC subgroups [64]. Among the MYD88 mutations, the L256P mutation is the most common mutation that simultaneously activates the JAK-STAT3 pathway [67, 68]. A mutation of CARD11 in GCB-subgroups can activate NF-κB even in the absence of antigen receptor signals (such as CD40-CD40L). Mutations in CD79A and CD79B are the most common ABC subtypes, and they are important components of BCR. CD79B and CD79A induce surface BCR expression through their effects on ITAM tyrosine residues [63]. BCL-6 and FOXP1 are the most common dysregulated genes of ABC subgroups. Additionally, NFKBIZ contributes to lymphomagenesis and is involved in the NF-κB and STAT3 pathways [69].
GCB subtype lesions
BCL-2 chromosomal translocation
In DLBCL, it is very common deregulate BCL-2 (18q21). The t (14;18) (q32; q21) translocation connects BCL-2 to the immunoglobulin heavy chain (IGHV) gene enhancer (14q32), resulting in BCL-2 deregulation [70,71,72]. The t (14;18) translocation occurs in 30%-40% of GCB subgroups [70], but it is not unique to DLBCL; rather, it occurs in 90% of follicular lymphomas. In the GCB subgroups, follicular lymphoma rarely presents in those younger than 18 years of age [73]. In the ABC subgroups, BCL-2 is rarely translocated (30%-40% of the cases), but it is more prone to gain or be amplified than in the GCB subgroups (15%) [74]. In GCB DLBCL, the promoter of BCL-2 is also frequently mutated [40, 61, 75], which is related to the presence of t (14;18). Although the prognosis of patients may be related to BCL-2 mutations and the treatment regimen adopted, technical biases might also impede the treatment effect [74, 76, 77]. Recently, two different large studies compared the effect of t (14;18) and BCL-2 on patient outcomes with R-CHOP, but only one study indicated that t (14;18) is related to poor outcomes in GCB patients [72]. Based on the results of both studies, BCL-2 is associated with poor prognosis in GCB DLBCL, but not in ABC DLBCL [72]. Contrary to previous studies, BCL-2 is only a poor prognostic factor for ABC subgroups [78].
EZH2
By sequencing and DNA profiling, EZH2 was found to be one of the most commonly mutated gene, occurring in approximately 6%-14% of DLBCL [39, 79,80,81]. It appears almost exclusively in the GCB subtypes, especially with BCL-2 translocations [81]. In 20% of GCB subgroups, EZH2 mutations are associated with t (14;18), but they are rarely seen in ABC DLBCL. Investigators believe that EZH2 inhibitors are considered promising preclinical data [82,83,84,85], and early relevant clinical trials are ongoing. DLBCL is accompanied by other gene mutations when chromatin modification occurs. Because of the low mutation rate and differences in the studied series, it is difficult to estimate the association with any specific subtype. There are several genes linked to DLBCL, including MLL2 (KMT2D) (22%-32% DLBCL), CREBBP (18%-20%), EP300 (5%-10%), and MLL3 (KMT2C) (15%) [79].
The lymphoma microenvironment (LME) can be divided into four distinct categories
Microenvironment cells and extracellular matrix (ECM) are responsible for external stimuli in the lymphoma niche, according to data obtained from lymphoma patients and animal models, leading to the development and progression of the disease, as well as the response to treatment [25, 86,87,88,89]. Due to bidirectional interactions between lymphoma cells and their microenvironment [90, 91], the complexity of the DLBCL microenvironment has yet to be defined. Although the DLBCL microenvironment has attracted increasing attention [25], people often only give attention to the disease itself during treatment and ignore the important role of the microenvironment [14]. DLBCL microenvironments vary in composition and functionality based on the gene expression profiles of thousands of patients. Twenty-five functional gene expression signatures (FGES) were discovered, reflecting either distinct cellular subtypes or noncellular components of the tumor microenvironment and activation of canonical signaling pathways in biological processes [92]. Nikita Kotlov et al. reported that the LME in DLBCL integrates characteristics of the microenvironment and malignant cells into the prognosis. We named the four distinct LMEs as follows: “germinal center-like” due to the presence of FGES from cell types commonly found in germinal centers (GC); “mesenchymal” (MS) refers to the abundance of FGES within stromal cells and intercellular matrix; “inflammatory” (IN) indicates FGES that are found in inflammatory cells or pathways; and “depleted” (DP) LMEs are characterized by an overall lower presence of FGES derived from the microenvironment [92]. Transcriptomic studies have found that the microenvironment correlates with disease biology [25, 30, 34]. Initial research focused on identifying differences in gene expression profiles among tumor samples [30, 34]. They extracted microenvironment signatures from transcriptomics to identify microenvironment cells in the transcriptome [93], and four distinct microenvironments reflecting unique biological characteristics and clinical behavior were proposed. As a result of these newly developed categories, we have identified distinct clinical behaviors among genetically similar DLBCLs and promising therapeutic targets [92].
Immunohistochemistry
The emergence of immunohistochemistry has met the increasing demand for personalized medicine, and the utility of applying complex genomics to clinical practice is clear. In recent years, according to the morphological review of the WHO classification of hematopoietic and lymphoid tissue tumors in 2017 and 2022, IHC is an important method for diagnosing and stratifying DLBCL [94]. Although there are many classification standards for DLBCL, the WHO mostly adopts the Hans criteria classification [95] (Table 3). IHC has always been considered one of the criteria for diagnosing DLBCL. Nevertheless, the Hans diagnostic criteria are approximately 80% consistent with gene expression profiling derived ABC-DLBCL and GCB-DLBCL classifications [95]. However, the accuracy of IHC diagnosis is challenged by GEP because there is an operation change of dyeing intensity in IHC. Nevertheless, with the widespread application of GEP and multi-genome platform analysis, IHC as an auxiliary tool for verifying genes is becoming increasingly important [96]. IHC can evaluate the degree of tumor infiltration [97], tumor microenvironment proteins [98], expression of tumor-promoting and tumor suppressor genes [99], and others. However, as the interpretation of IHC results varies by person, it is difficult to use it as the only criterion for disease diagnosis. With the advent of genomics and other new computational tools, the importance of IHC has been gradually weakened [96]. Another important role of IHC is to evaluate the prognosis of patients, especially in patients with double-expression, that is, ≥ 40% MYC and ≥ 50% BCL-2 are simultaneously expressed in lymphoma cells [100]. The researchers established a correlation with the double expression lymphoma score, which can effectively predict the inferior outcome of these patients; other studies have also supported this idea [101,102,103].
Diagnosis and staging
Molecular diagnosis
The molecular classification of DLBCL requires an excisional biopsy and expert hematopathologist review to ensure adequate tissue available for diagnostic assessment [109]. When the excisional biopsy cannot recognize the tumor type, a core biopsy is required [110, 111]. The diagnosis of DLBCL is based on the WHO 2022 criteria [4]. Somatic mutation and intraclonal variation in the V region of the Ig gene are characteristic changes in GCB cells [112, 113]. BCL-6 and CD10 are markers of germinal center B-cells, while IRF4/MUM1 is mainly expressed in the late stage of plasma cell and B-cell development and is a marker of non-GCB [114, 115]. IRF4 is transiently expressed when activated by normal lymphocytes and participates in the proliferative response of B-cells after antigen activation [116,117,118]. During ABC-type cell proliferation and tumor formation, IRF4 plays an important role in constitutive expression [119, 120]. Therefore, DLBCL-not other specified (DLBCL-NOS) can be classified as GCB according to CD10, BCL-6 and IRF4/MUM1 and non-GCB [121]. GCB can be diagnosed in the following cases: CD10 is positive; CD10 is negative, but BCL-6 is positive and IRF4/MUM1 is negative, and the others are non-GCB [122, 123].
Gene expression analysis showed that the t (14; 18) (q32; q21) translocation involves the BCL-2 gene and is found only in GCB subtypes [124]. The 3q27 translocation involving BCL-6 can be found in 30% ~ 40% of DLBCL cases. The expression of BCL-6 plays a significant role in the development of the germinal center and the response to antigens; thus, it is known as a germinal center marker [125]. It has been reported that BCL-6 can inhibit the expression of PRDM1, which is an important regulatory gene for plasma cell differentiation [126]. In addition, inhibiting the normal downregulation of BCL-6 leads to cell differentiation arrest and continued proliferation, thus leading to tumorigenesis [127]. It has also been shown that abnormal chromosome translocation results in the deregulation of BCL-6, which inhibits the downregulation of BCL-6 expression, causing abnormal expression of BCL-6 in some non-GCB DLBCL subgroups [128].
Aberrant activation of the NF-κB pathway is a feature of ABC subtypes. The activation of NF-κB caused by the excessive activity of IKK leads to rapid IκB degradation by pantothenate proteasome, resulting in NF-κB release and translocation to the nucleus to activate a series of transcription factors. This promotes cell proliferation and inhibits apoptosis, which results in long-term tumor cell survival [129, 130]. Because constitutive activation of IKK is a unique feature of ABC subtypes, NF-κB may be a new potential treatment target for ABC subtypes, and it has been confirmed that inhibition of IKK activity can promote apoptosis of ABC subtypes but not GCB subtypes [131]. In addition, ABC and GCB also have obvious differences in response to IL-4 [132]. IL-4 promotes GCB subtypes to induce high expression of downstream target genes, such as BCL-6, through activating signal transcription activator 6 (STAT6 phosphorylation) and ultimately promotes cell proliferation [133]. This may explain why ABC-DLBCL is not sensitive to cell cycle drugs. We summarize the differences.
between GCB and ABC in Table 4.
Other adjunctive diagnoses
In some selected circumstances, bone marrow biopsy (BMB) remains an important diagnostic method for DLBCL. The clinical manifestation, organ function evaluation and Ann Arbor score of patients are also essential as important auxiliary diagnostic methods. PET-CT combines the benefits of PET and contrast-enhanced CT and should therefore be recommended for all DLBCL patients for diagnosis and efficacy evaluation; importantly, it can identify more DLBCL cells than a standard contrast-enhanced CT alone, with PET staging in 5% to 15% of DLBCL [134, 135]. A superior option in Lugano staging recommendations is BMB, which has shown valuable in the PET era [134]. DLBCL is widely diagnosed using PET-CT, which provides high sensitivity and specificity. However, indolent or low-volume disease may go undetected [136]. Thus, BMB is still the most accurate, reliable and irreplaceable diagnostic method for DLBCL.
Treatment
The R-CHOP regimen can cure 60% of DLBCL patients [137, 138]. However, with the continuous development of diagnosis and treatment technology, more individualized treatment should also be widely used. For elderly patients with poor basic conditions, the chemotherapy cycles and times can be shortened according to the disease location and scope to reduce the chemotherapy risk. The four treatment regimens based on rituximab are the main regimens for the treatment of DLBCL at this stage.
Combination therapy: chemotherapy plus involved-site radiotherapy (ISRT)
In bulky (≥ 7.5 cm) DLBCL patients, radiotherapy as a treatment for the consolidation phase after chemotherapy can bring benefits to patients. Among non- bulky (< 7.5 cm) DLBCL patients, patients with limited disease duration and smIPI score ≥ 1 received a 3-cycle RCHOP regimen combined with 40–46 Gy doses of radiotherapy. The PFS for 2 and 4 years is 93% and 88%, respectively. 95% OS at 2 years, 92% at 4 years [139]. In another experiment comparing RCHOP and RCHOP-RT, it was found that there was no statistically significant difference in 5-year EFS between the two groups. The R-CHOP group had 89% ± 2.9%, while the R-CHOP combined with RT group had 92% ± 2.4%. The OS of patients receiving R-CHOP treatment alone was 92% (95% CI, 89.5% -94.5%), while R-CHOP-RT was 96% (95% CI, 94.3%-97.7%) (P not significant). Therefore, in non-bulky DLBCL patients, the benefits of chemotherapy combined with radiotherapy are not significant [138]. In addition, some special extranodal DLBCL, such as CNS DLBCL, with primary ocular involvement, localized skin involvement, testicular involvement, etc., are also recommended for radiation therapy during the consolidation phase [140].
Standard R-CHOP
The R-CHOP regimen was found to be effective in treating DLBCL patients aged 18 to 60 years, with a favorable overall survival (OS) rate after combining rituximab. Seventy-two percent of patients were in stages I to II, and only 3% had a baseline mass greater than 10 cm. Compared with chemotherapy alone, the combination of rituximab improved the OS of patients from 80 to 90% at six years. Patients with a mass size < 5 cm and without other IPI risk factors had the best outcomes, with 95% OS at 6 years [141]. The effectiveness of R-CHOP is equivalent to that of combined modality treatment (CMT), avoiding RT by enhancing systemic therapy compared to whole-course R-CHOP [142, 143]. However, some researchers found that, after 6 to 8 cycles of R-CHOP chemotherapy plus ISRT, the progression-free survival (PFS) and OS of patients were improved [144]. In general, the above results showed that conventional ISRT after whole-course R-CHOP treatment has certain benefits, but the side effects of ISRT are often found decades after treatment. The use of R-CHOP alone for 6 cycles has proven safe and effective in the treatment of DLBCL, especially in patients with high-risk diseases. This type of patient includes: stage I and II (excluding stage II with extensive mesenteric diseases) with or without large masses (≥ 7.5 cm). Clinical practitioners are also striving to tailor treatment programs based on the patient’s conditions to promote the concept of individualized treatment.
R-CHOP plus ‘X’
Based on the original standard first-line treatment scheme, R-CHOP + ‘X’ has become increasingly popular with patients over time. Figure 2 illustrates the mechanism by which the R-CHOP regimen plus ‘X’ drugs are used in treating DLBCL.
Mechanism of R-CHOP regimen plus ‘X’ drugs in treatment of DLBCL. The backbone R-CHOP has been combined with a number of add-on therapies. The immunomodulatory effect of malidomide is mediated by the regulation of T/NK cells, Venetoclax blocks anti-apoptotic protein BCL-2, Lenalidomide is an immunomodulant agent that blocks cereblon, Bortezomib inhibits proteasomes, Ibrutinib inhibits Bruton Tyrosine Kinase, and Polatuzumab inhibits CD79b
In phase II single arm trials of lenalidomide with R-CHOP (R2-CHOP), the drug showed promise as a frontline therapy for non-GCB DLBCL [145,146,147]. Consequently, R2-CHOP was subsequently tested for DLBCL in two randomized studies in comparison to R-CHOP. A phase II randomized clinical trial involving 349 patients demonstrated a positive difference in both OS and PFS for patients with the ABC subtype of DLBCL treated with R2-CHOP [148]. In a phase III trial, consisting of 570 ABC-DLBCL patients, lenalidomide was added with a slightly different schedule from the previous study, although patients with high-risk diseases (IPI score 3 or more) showed a trend favoring R2-CHOP over placebo/RCHOP, the PFS did not improve [149].
The proteasome inhibitor Bortezomib was also unable to improve outcomes over R-CHOP in the phase II PYRAMID study or in the phase III REMoDL-B study. In the latter study, in which patients were also stratified based on their COO, no differences were observed between the two arms [150]. Similarly, when added to R-CHOP (RB-CHOP), the proteasome inhibitor Bortezomib failed to improve outcomes compared to R-CHOP in phase II PYRAMID and phase III REMoDL-B phase III trial, a subsequent study that also stratified patients by COO did not find any differences between the two arms [150]. In patients with double-hit lymphoma, PFS at 30 months is higher after R-CHOP in comparison to RB-CHOP at 58.8%, although this was derived from a post-hoc analysis, and the difference was not statistically significant [151].
Ibrutinib is an oral inhibitor of Bruton’s tyrosine kinase (BTK) and has been approved for several B-cell malignancies, including R/R ABC DLBCL, possibly related to the chronic activation of B-cell receptor and NF-κB patterns which characterize this COO subtype [148]. However, in the phase III PHOENIX trial, ibrutinib plus R-CHOP was compared to placebo + RCHOP, but neither of the primary or secondary endpoints were significantly improved [151]. A pre-planned exploratory analysis identified a significant interaction between treatment and age: when administered to patients under 60 years of age, ibrutinib plus R-CHOP resulted in improved outcomes with manageable safety, but when given to older patients, the addiction to ibrutinib led to adverse effects and compromised treatment administration [151]. With the purpose of ameliorate PHOENIX results, ESCALADE (NCT04529772) is a phase III trial randomized to perform R-CHOP or R-CHOP plus acalabrutinib on young untreated non-GCB DLBCL patients (65 years old), a selective second-generation BTK inhibitor with fewer off-target side effects [151].
Pola + R-CHP
Polatuzumab vedotin is an antibody–drug conjugate that combines monoclonal antibodies targeting CD79b, a cell-surface antigen expressed exclusively on mature B cells except plasma cells, with monomethyl auristatin E, a cytotoxic agent. Since 2021, public health insurance systems in Japan have approved and covered polatuzumab vedotin for the treatment of relapsed or refractory DLBCL [151]. Pola + R-CHP (polatuzumab vedotin plus rituximab, cyclophosphamide, doxorubicin, and prednisolone) combination therapy was evaluated in a phase III, multi-institutional, randomized, double-blind, placebo-controlled trial (POLARIX: GO39942). A study showing superiority of Pola + R-CHP over CHOP therapy for previously untreated CD20-positive DLBCL with an IPI score of 2 showed that Pola + R-CHP delivered superior PFS (A risk ratio of 0.73 [95% CI: 0.57–0.95; p = 0.02] was obtained for progression, relapse, or death) as compared with R-CHOP regimens. Despite this, OS did not differ significantly between the groups (hazard ratio for death = 0.94 [95%CI: 0.65–1.37; p = 0.75]). According to data from the POLARIX trial and other studies, Pola + R-CHP was approved by the Japan Ministry of Health and Welfare in August 2022 [151].
PET-adapted therapy
PET was used as an important auxiliary tool for the diagnosis of DLBCL, which filled the gap in imaging, with the following three objectives staging, prognosis evaluation, and response to treatment. Disease staging by PET can find additional sites of lesions in 35% of patients, and 12% of patients have higher stages [152]. A retrospective study of prognosis found that 56% of the positive predictive value had an IPI < 3, compared with 80% for patients with an IPI ≥ 3. Using PET to monitor disease recurrence, the accuracy rate can reach more than 95% [153]. Generally, negative PET indicates a good prognosis, and CT re-examination may not be required in a short time [154]. The treatment plan of the British Columbia Cancer Agency (BCCA) for DLBCL patients is that the patients receive three cycles of R-CHOP treatment achieving a complete remission (CR) by PET and then receive an additional cycle of R-CHOP treatment. To better clear residual lesions, ISRT is also acceptable. Approximately 80% of the studied population had at least one risk factor for stage-modified IPI (smIPI). The 3-year PFS and 3-year OS of patients with negative interim PET (iPET) results were 92% and 96%, respectively, while the 3-year PFS and OS of patients with positive iPET results were 60% and 83%, respectively [155, 156]. This study showed that the time and related toxicity of chemoimmunotherapy can be reduced by using iPET to evaluate the therapeutic effect, while patients with iPET positivity still need to optimize treatment. Another study further evaluated whether R-CHOP at 6 cycles after PET imaging was better than that at 4 cycles. The initial treatment of DLBCL patients with R-CHOP was two cycles, those with iPET-negative tumors received only four cycles, and those with iPET-positive tumors received a total of six cycles. After 5 years of follow-up, all patients treated with R-CHOP achieved 92% PFS in the experimental group and 89% PFS in the standard group at 3 years [157]. Therefore, it can effectively evaluate the patient’s condition, select different treatment regimens, shorten the treatment cycle and reduce the treatment risk. Their research also discovered the role of other PET-derived biomarkers, such as metabolic tumor volume, which are predictive of PFS [158] and OS [159]. Research from Wyndham H. Wilson et al. Showed that ibrutinib with R-CHOP could increase event-free survival (EFS) of patients with MCD DLBCL from 48% to 69.6% [160].
Treatment of relapsed refractory DLBCL
Clinical trials are first recommended for relapsed or refractory (R/R) DLBCL. For patients who R/R to their first-line therapy, salvage high-dose chemotherapy and an autologous stem-cell transplant (ASCT) are the standard second-line treatments [161, 162] (Fig. 3). The strategy, however, is beneficial only to healthy patients without comorbidities [161]. Furthermore, studies have shown that even intensive therapy can fail to improve the outcome of patients with primary refractory disease or patients who are relapsing within 12 months of first-line therapy, with an objective response rate (ORR) of 26%, a CR rate of 7%, and a median OS rate of 6.3 months were achieved [163].
CAR t-cell therapy
Newly authorized treatment choices, like chimeric antigen receptor T cell (CAR-T) therapy, have been recently approved. Polatuzumab vedotin, tafasitamab in combination with lenalidomide, loncastuximab tesirine, or selinexor could be potential treatment choices for individuals with R/R DLBCL, particularly for those who have undergone two or more lines of therapy (LOTs) and/or are not suitable candidates for autologous stem cell transplantation (ASCT) [164,165,166,167]. Clinical trials of CAR-T in phase 1/2 reported an ORR of 52 to 82% [168,169,170]. More recently, clinical trials testing CAR-T therapies against salvage therapy with the intention of combining with HDT-ASCT have demonstrated significant benefits among patients suffering from primary refractory DLBCL or who have relapsed within 12 months of receiving 1line therapy, this represents an important step forward for patients with R/R DLBCL [171]. Although CAR-T therapy may be effective for some patients, it has been plagued with serious toxicities, high rates of disease progression, and limited eligibility for treatment [172, 173]. In patients with R/R cancer, CAR T-cell therapy is a superior treatment option. CD19 is the first approved product that involves autologous T cells. In early clinical trials, the overall response and CR rates of relapsed and refractory patients after treatment with axicabtagene ciloleucel, tisagenlecleucel, and lisocabtagene maraleucel were 52 to 82% and 40 to 54%, respectively [168,169,170]. In subsequent studies, 37% of patients had a median survival of 27 months after receiving axicabtagene ciloleucel [174]. Of course, there are errors in the experimental results because patients receiving treatment are all selected. Because of its side effects, CAR T-cell therapy is not suitable for all patients. The investigators found that, after the patients received CAR T-cell therapy, the incidence of grade 3–4 cytokine release syndrome and neurotoxic effects was 2–22% and 10–28%, respectively [168,169,170]. At present, the wide use of CAR T-cell therapy is limited by various factors, such as large toxicity and side effects, high economic costs and the disease process of patients [175]. Therefore, it is urgent to develop multitarget and allogeneic off-the-shelf products to provide more choices for patients in the future. Figure 4 shows the pattern diagram of CAR-Ts. Some small molecule targeted drugs, such as ABT-199, a selective inhibitor of BCL-2, lenalidomide, a tyrosine kinase inhibitor, and an epigenetic regulator (EZH2 inhibitor tazemetostat), have been applied in the clinic as an important part of the combined treatment regimen [151, 176,177,178]. In addition, pathway-based approaches should be taken seriously, such as NOTCH, JAK-STAT, and PI3K-AKT-mTOR [179]. The novel perspectives and breakthroughs in the treatment of DLBCL are listed in Table 5.
Targeting antigen-expressing tumor cells with CAR T cells. T cells transduced with viral particles harboring the CAR-encoding transgene express CARs on their surfaces in a stable manner. The activation of CAR-T cells occurs when they encounter a tumor antigen, releasing perforin and granzymes that cause the tumor cells to die
Checkpoint inhibitors
-
1.
Cytotoxic T lymphocyte-associated antigen-4 (CTLA-4)
Both CD4+ and CD8+ T cells express the homologous receptors CD28 and CTLA4. Activation of T cells is mediated by the opposing effects of these receptors. The T-cell-mediated immune response is activated by CD28, while the T-cell-mediated immune response is suppressed by CTLA-4. Ipilimumab, the first anti-CTLA-4 monotherapy has achieved significant clinical effects since in 2011. The most striking observation regarding ipilimumab was the increase in overall patient survival of up to 10 years for some patients [189, 190].
-
2.
Programmed cell death (PD-1)
There is a 20% sequence homology between PD-1, which is also known as CD279, and CTLA4, which was discovered in 1992. As an inhibitor of both adaptive and innate immune responses. In addition, PD-1 has sustained expression during persistent antigen encounters, which limits protective immunity. T cells are not the only cells expressing PD-1 during persistent antigen encounters, and the phenomenon can be observed both in hematopoietic and nonhematopoietic cells. Thus, PD-1 plays an important role in secondary lymphoid organ immune cell function [191, 192].
Next-generation immune checkpoint targets
It is expected that an increasing number of immune checkpoint targets will be developed as medical technology advances, including LAG-3 (CD223), B7-H3 and B7-H4, A2aR and CD73, and NKG2A.
Conclusions
In this review, we summarize the genetic events of DLBCL and how they promote the development of this type of lymphoma and discuss the clinical importance of genetic abnormalities. The application of genetics, immunology and TME in the classification, diagnosis and treatment of DLBCL is helpful to better understand the biology of lymphoma. Several elegant studies have uncovered the functional implications of genetic aberrations, including those involving BCL-6, CREBBP, KMT2D and others. However, the exact functional relevance of many genetic aberrations remains unclear. There is limited information available at present regarding the stages of B-cell maturation during which these aberrations occur. Genetic and pathway mutations recurrent in DLBCL reveal vulnerabilities in lymphoma cells that are often associated with distinct lymphoma subtypes, and more effective, targeted therapeutic approaches could be developed. The findings from these studies are already being applied to the development of products, services and novel drugs or drug combinations being tested (or repositioned) in DLBCL to combat specific dysregulated program. The different diagnostic criteria of DLBCL are described in detail. Finally, the treatment progress of DLBCL was summarized. The latest description of the genetics, biology and diagnostics of DLBCL will help to develop new and, more importantly, accurate treatment methods for patients with DLBCL.
Data availability
Not applicable.
Abbreviations
- DLBCLs:
-
Diffuse large B-cell lymphomas
- GEP:
-
Gene-expression profile
- ABC:
-
Activated B-cell
- GCB:
-
Germinal-center B-cell
- R-CHOP:
-
Rituximab Cyclophosphamide, Doxorubicin, Vincristine, Prednisone; COO: Cell-of-origin
- LME:
-
Lymphoma microenvironment
- RT:
-
Radiotherapy
- BCL-2:
-
B-cell lymphoma-2
- CAR-T:
-
Chimeric antigen receptor T cell immunotherapy
- NHL:
-
Non-Hodgkin lymphoma
- JAK:
-
Janus kinase
- NF-Κb:
-
Nuclear factor-kB
- MYD88:
-
Myeloid differentiation primary response 88
- PFS:
-
Progression-free survival
- STAT3:
-
Signal transducer and activator of transcription 3
- BCR:
-
B-cell receptor
- BTK:
-
Bruton tyrosine kinase
- NK cell:
-
Natural killer cell
- scFv:
-
Single-chain variable fragment
- TM:
-
Transmembrane domain
- CNS:
-
Central nervous system
- DA-EPOCH-CHOP:
-
Dose-adjusted-etoposide, Prednisone, Vincristine, Cyclophosphamide and Doxorubicin- Cyclophosphamide, Doxorubicin, Vincristine and Prednisone
- XPO1:
-
Exportin 1
- ALK:
-
Anaplastic lymphoma kinase
- IRF4:
-
Interferon regulatory factor 4
- BCL-6:
-
B-cell lymphoma-6
- NOS:
-
Not otherwise specified
- PMBL:
-
Primary mediastinal large B-cell lymphoma
- FAD:
-
Food drug administration
- OSR:
-
5- Or 3-year overall survival rate
- EFS:
-
Event-free survival
- ASCT:
-
Autologous stem-cell transplant
- CR/PR:
-
Complete remission/partial remission
- ORR:
-
Objective response rat
- CR:
-
Complete remission
- OS:
-
Overall survival
References
Testoni M et al (2015) Genetic lesions in diffuse large B-cell lymphomas. Ann Oncol 26(6):1069–1080
Roschewski M, Staudt LM, Wilson WH (2014) Diffuse large B-cell lymphoma-treatment approaches in the molecular era. Nat Rev Clin Oncol 11(1):12–23
Coiffier B et al (2002) CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med 346(4):235–242
Alaggio R et al (2022) The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia 36(7): 1720–1748
Martinez-Climent JA et al (2003) Transformation of follicular lymphoma to diffuse large cell lymphoma is associated with a heterogeneous set of DNA copy number and gene expression alterations. Blood 101(8):3109–3117
Kridel R et al (2017) Predicting transformation of follicular lymphoma. Blood 130(3):258–266
Bouska A et al (2014) Genome-wide copy-number analyses reveal genomic abnormalities involved in transformation of follicular lymphoma. Blood 123(11):1681–1690
Pasqualucci L et al (2014) Genetics of follicular lymphoma transformation. Cell Rep 6(1):130–140
Rossi D, Gaidano G (2009) Richter syndrome: molecular insights and clinical perspectives. Hematol Oncol 27(1):1–10
Chigrinova E et al (2013) Two main genetic pathways lead to the transformation of chronic lymphocytic leukemia to Richter syndrome. Blood 122(15):2673–2682
Tiirikainen MI et al (2001) DNA copy number alterations in HIV-positive and HIV-negative patients with diffuse large-cell lymphomas. J Acquir Immune Defic Syndr 27(3):272–276
Gucalp A, Noy A (2010) Spectrum of HIV lymphoma 2009. Curr Opin Hematol 17(4):362–367
Carbone A et al (2001) Genetic pathways and histogenetic models of AIDS-related lymphomas. Eur J Cancer 37(10):1270–1275
Frontzek F, Lenz G (2019) Novel insights into the pathogenesis of molecular subtypes of diffuse large B-cell lymphoma and their clinical implications. Expert Rev Clin Pharmacol 12(11):1059–1067
Chapuy B et al (2018) Molecular subtypes of diffuse large B cell lymphoma are associated with distinct pathogenic mechanisms and outcomes. Nat Med 24(5):679–690
Schmitz R et al (2018) Genetics and Pathogenesis of Diffuse Large B-Cell Lymphoma. N Engl J Med 378(15):1396–1407
Wright GW et al (2020) A Probabilistic Classification Tool for Genetic Subtypes of Diffuse Large B Cell Lymphoma with Therapeutic Implications. Cancer Cell 37(4):551-568 e14
Barraclough A et al (2019) COO and MYC/BCL2 status do not predict outcome among patients with stage I/II DLBCL: a retrospective multicenter study. Blood Adv 3(13):2013–2021
Kumar A et al (2015) Excellent outcomes and lack of prognostic impact of cell of origin for localized diffuse large B-cell lymphoma in the rituximab era. Br J Haematol 171(5):776–783
Cunningham D et al (2013) Rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone in patients with newly diagnosed diffuse large B-cell non-Hodgkin lymphoma: a phase 3 comparison of dose intensification with 14-day versus 21-day cycles. Lancet 381(9880):1817–1826
Scott DW et al (2015) Prognostic Significance of Diffuse Large B-Cell Lymphoma Cell of Origin Determined by Digital Gene Expression in Formalin-Fixed Paraffin-Embedded Tissue Biopsies. J Clin Oncol 33(26):2848–2856
Augustyn A et al (2021) The impact of cell-of-origin, MYC/Bcl-2 dual expression and MYC rearrangement on disease relapse among early stage diffuse large B-cell lymphoma patients treated with combined modality therapy. Leuk Lymphoma 62(6):1361–1369
Johnson NA et al (2009) Lymphomas with concurrent BCL2 and MYC translocations: the critical factors associated with survival. Blood 114(11):2273–2279
Scott DW et al (2018) High-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements with diffuse large B-cell lymphoma morphology. Blood 131(18):2060–2064
Lenz G et al (2008) Stromal gene signatures in large-B-cell lymphomas. N Engl J Med 359(22):2313–2323
Alizadeh AA et al (2000) Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 403(6769):503–511
Wright G et al (2003) A gene expression-based method to diagnose clinically distinct subgroups of diffuse large B cell lymphoma. Proc Natl Acad Sci USA 100(17):9991–9996
Rosenwald A et al (2003) Molecular diagnosis of primary mediastinal B cell lymphoma identifies a clinically favorable subgroup of diffuse large B cell lymphoma related to Hodgkin lymphoma. J Exp Med 198(6):851–862
Lenz G, Staudt LM (2010) Aggressive lymphomas. N Engl J Med 362(15):1417–1429
Rosenwald A et al (2002) The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med 346(25):1937–1947
Visco C et al (2012) Comprehensive gene expression profiling and immunohistochemical studies support application of immunophenotypic algorithm for molecular subtype classification in diffuse large B-cell lymphoma: a report from the International DLBCL Rituximab-CHOP Consortium Program Study. Leukemia 26(9):2103–2113
Guo L et al (2018) Molecular heterogeneity in diffuse large B-cell lymphoma and its implications in clinical diagnosis and treatment. Biochim Biophys Acta Rev Cancer 1869(2):85–96
Wilson WH et al (2012) A Cancer and Leukemia Group B multi-center study of DA-EPOCH-rituximab in untreated diffuse large B-cell lymphoma with analysis of outcome by molecular subtype. Haematologica 97(5):758–765
Monti S et al (2005) Molecular profiling of diffuse large B-cell lymphoma identifies robust subtypes including one characterized by host inflammatory response. Blood 105(5):1851–1861
Alexandrov LB et al (2013) Signatures of mutational processes in human cancer. Nature 500(7463):415–421
Lawrence MS et al (2013) Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 499(7457):214–218
Morin RD et al (2013) Mutational and structural analysis of diffuse large B-cell lymphoma using whole-genome sequencing. Blood 122(7):1256–1265
Pasqualucci L et al (2011) Analysis of the coding genome of diffuse large B-cell lymphoma. Nat Genet 43(9):830–837
Morin RD et al (2011) Frequent mutation of histone-modifying genes in non-Hodgkin lymphoma. Nature 476(7360):298–303
Saito M et al (2009) BCL6 suppression of BCL2 via Miz1 and its disruption in diffuse large B cell lymphoma. Proc Natl Acad Sci USA 106(27):11294–11299
Pasqualucci L et al (2001) Hypermutation of multiple proto-oncogenes in B-cell diffuse large-cell lymphomas. Nature 412(6844):341–346
Cattoretti G et al (2005) Deregulated BCL6 expression recapitulates the pathogenesis of human diffuse large B cell lymphomas in mice. Cancer Cell 7(5):445–455
Iqbal J et al (2007) Distinctive patterns of BCL6 molecular alterations and their functional consequences in different subgroups of diffuse large B-cell lymphoma. Leukemia 21(11):2332–2343
Saito M et al (2007) A signaling pathway mediating downregulation of BCL6 in germinal center B cells is blocked by BCL6 gene alterations in B cell lymphoma. Cancer Cell 12(3):280–292
Juszczynski P et al (2009) BCL6 modulates tonic BCR signaling in diffuse large B-cell lymphomas by repressing the SYK phosphatase. PTPROt Blood 114(26):5315–5321
Basso K et al (2012) BCL6 positively regulates AID and germinal center gene expression via repression of miR-155. J Exp Med 209(13):2455–2465
Chen YW et al (2006) High BCL6 expression predicts better prognosis, independent of BCL6 translocation status, translocation partner, or BCL6-deregulating mutations, in gastric lymphoma. Blood 108(7):2373–2383
Ott G et al (2010) Immunoblastic morphology but not the immunohistochemical GCB/nonGCB classifier predicts outcome in diffuse large B-cell lymphoma in the RICOVER-60 trial of the DSHNHL. Blood 116(23):4916–4925
Horn H et al (2013) MYC status in concert with BCL2 and BCL6 expression predicts outcome in diffuse large B-cell lymphoma. Blood 121(12):2253–2263
Winter JN et al (2006) Prognostic significance of Bcl-6 protein expression in DLBCL treated with CHOP or R-CHOP: a prospective correlative study. Blood 107(11):4207–4213
Oki Y et al (2008) Prognostic value of serum soluble interleukin-2 receptor level in patients with diffuse large B cell lymphoma, treated with CHOP- or RCHOP-based therapy. Leuk Lymphoma 49(7):1345–1351
Akyurek N et al (2012) Prognostic significance of MYC, BCL2, and BCL6 rearrangements in patients with diffuse large B-cell lymphoma treated with cyclophosphamide, doxorubicin, vincristine, and prednisone plus rituximab. Cancer 118(17):4173–4183
Copie-Bergman C et al (2009) Immuno-fluorescence in situ hybridization index predicts survival in patients with diffuse large B-cell lymphoma treated with R-CHOP: a GELA study. J Clin Oncol 27(33):5573–5579
Mandelbaum J et al (2010) BLIMP1 is a tumor suppressor gene frequently disrupted in activated B cell-like diffuse large B cell lymphoma. Cancer Cell 18(6):568–579
Compagno M et al (2009) Mutations of multiple genes cause deregulation of NF-kappaB in diffuse large B-cell lymphoma. Nature 459(7247):717–721
Kato M et al (2009) Frequent inactivation of A20 in B-cell lymphomas. Nature 459(7247):712–716
Calado DP et al (2010) Constitutive canonical NF-kappaB activation cooperates with disruption of BLIMP1 in the pathogenesis of activated B cell-like diffuse large cell lymphoma. Cancer Cell 18(6):580–589
Lenz G et al (2008) Molecular subtypes of diffuse large B-cell lymphoma arise by distinct genetic pathways. Proc Natl Acad Sci USA 105(36):13520–13525
Lenz G et al (2007) Aberrant immunoglobulin class switch recombination and switch translocations in activated B cell-like diffuse large B cell lymphoma. J Exp Med 204(3):633–643
Shaffer AL et al (2002) Blimp-1 orchestrates plasma cell differentiation by extinguishing the mature B cell gene expression program. Immunity 17(1):51–62
Lohr JG et al (2012) Discovery and prioritization of somatic mutations in diffuse large B-cell lymphoma (DLBCL) by whole-exome sequencing. Proc Natl Acad Sci USA 109(10):3879–3884
Lenz G et al (2008) Oncogenic CARD11 mutations in human diffuse large B cell lymphoma. Science 319(5870):1676–1679
Davis RE et al (2010) Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma. Nature 463(7277):88–92
Ngo VN et al (2011) Oncogenically active MYD88 mutations in human lymphoma. Nature 470(7332):115–119
Cao R et al (2002) Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science 298(5595):1039–1043
Bonetti P et al (2013) Deregulation of ETS1 and FLI1 contributes to the pathogenesis of diffuse large B-cell lymphoma. Blood 122(13):2233–2241
Scuto A et al (2011) STAT3 inhibition is a therapeutic strategy for ABC-like diffuse large B-cell lymphoma. Cancer Res 71(9):3182–3188
Ding BB et al (2008) Constitutively activated STAT3 promotes cell proliferation and survival in the activated B-cell subtype of diffuse large B-cell lymphomas. Blood 111(3):1515–1523
Nogai H et al (2013) IkappaB-zeta controls the constitutive NF-kappaB target gene network and survival of ABC DLBCL. Blood 122(13):2242–2250
Iqbal J et al (2004) BCL2 translocation defines a unique tumor subset within the germinal center B-cell-like diffuse large B-cell lymphoma. Am J Pathol 165(1):159–166
Iqbal J et al (2011) BCL2 predicts survival in germinal center B-cell-like diffuse large B-cell lymphoma treated with CHOP-like therapy and rituximab. Clin Cancer Res 17(24):7785–7795
Visco C et al (2013) Patients with diffuse large B-cell lymphoma of germinal center origin with BCL2 translocations have poor outcome, irrespective of MYC status: a report from an International DLBCL rituximab-CHOP Consortium Program Study. Haematologica 98(2):255–263
Klapper W et al (2012) Patient age at diagnosis is associated with the molecular characteristics of diffuse large B-cell lymphoma. Blood 119(8):1882–1887
Kendrick SL et al (2014) BCL2 antibodies targeted at different epitopes detect varying levels of protein expression and correlate with frequent gene amplification in diffuse large B-cell lymphoma. Hum Pathol 45(10):2144–2153
Schuetz JM et al (2012) BCL2 mutations in diffuse large B-cell lymphoma. Leukemia 26(6):1383–1390
de Jong D et al (2009) Immunohistochemical prognostic markers in diffuse large B-cell lymphoma: validation of tissue microarray as a prerequisite for broad clinical applications (a study from the Lunenburg Lymphoma Biomarker Consortium). J Clin Pathol 62(2):128–138
de Jong D et al (2007) Immunohistochemical prognostic markers in diffuse large B-cell lymphoma: validation of tissue microarray as a prerequisite for broad clinical applications–a study from the Lunenburg Lymphoma Biomarker Consortium. J Clin Oncol 25(7):805–812
Iqbal J et al (2006) BCL2 expression is a prognostic marker for the activated B-cell-like type of diffuse large B-cell lymphoma. J Clin Oncol 24(6):961–968
Pasqualucci L et al (2011) Inactivating mutations of acetyltransferase genes in B-cell lymphoma. Nature 471(7337):189–195
Zhang J et al (2013) Genetic heterogeneity of diffuse large B-cell lymphoma. Proc Natl Acad Sci USA 110(4):1398–1403
Morin RD et al (2010) Somatic mutations altering EZH2 (Tyr641) in follicular and diffuse large B-cell lymphomas of germinal-center origin. Nat Genet 42(2):181–185
McCabe MT et al (2012) Mutation of A677 in histone methyltransferase EZH2 in human B-cell lymphoma promotes hypertrimethylation of histone H3 on lysine 27 (H3K27). Proc Natl Acad Sci USA 109(8):2989–2994
Qi W et al (2012) Selective inhibition of Ezh2 by a small molecule inhibitor blocks tumor cells proliferation. Proc Natl Acad Sci USA 109(52):21360–21365
McCabe MT et al (2012) EZH2 inhibition as a therapeutic strategy for lymphoma with EZH2-activating mutations. Nature 492(7427):108–112
Knutson SK et al (2012) A selective inhibitor of EZH2 blocks H3K27 methylation and kills mutant lymphoma cells. Nat Chem Biol 8(11):890–896
Kant S, Kumar A, Singh SM (2014) Tumor growth retardation and chemosensitizing action of fatty acid synthase inhibitor orlistat on T cell lymphoma: implication of reconstituted tumor microenvironment and multidrug resistance phenotype. Biochim Biophys Acta 1840(1):294–302
Cayrol F et al (2015) Integrin alphavbeta3 acting as membrane receptor for thyroid hormones mediates angiogenesis in malignant T cells. Blood 125(5):841–851
Ciavarella S et al (2019) Dissection of DLBCL microenvironment provides a gene expression-based predictor of survival applicable to formalin-fixed paraffin-embedded tissue. Ann Oncol 30(12):2015
Ciavarella S et al (2018) Dissection of DLBCL microenvironment provides a gene expression-based predictor of survival applicable to formalin-fixed paraffin-embedded tissue. Ann Oncol 29(12):2363–2370
de Charette M, Houot R (2018) Hide or defend, the two strategies of lymphoma immune evasion: potential implications for immunotherapy. Haematologica 103(8):1256–1268
Pandey S et al (2017) IL-4/CXCL12 loop is a key regulator of lymphoid stroma function in follicular lymphoma. Blood 129(18):2507–2518
Kotlov N et al (2021) Clinical and Biological Subtypes of B-cell Lymphoma Revealed by Microenvironmental Signatures. Cancer Discov 11(6):1468–1489
Becht E et al (2016) Estimating the population abundance of tissue-infiltrating immune and stromal cell populations using gene expression. Genome Biol 17(1):218
Sabattini E et al (2010) WHO classification of tumours of haematopoietic and lymphoid tissues in 2008: an overview. Pathologica 102(3):83–87
Hans CP et al (2004) Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood 103(1):275–282
Opinto G et al (2020) The Tumor Microenvironment of DLBCL in the Computational Era. Front Oncol 10:351
Keane C et al (2013) CD4(+) tumor infiltrating lymphocytes are prognostic and independent of R-IPI in patients with DLBCL receiving R-CHOP chemo-immunotherapy. Am J Hematol 88(4):273–276
Meyer PN et al (2011) The stromal cell marker SPARC predicts for survival in patients with diffuse large B-cell lymphoma treated with rituximab. Am J Clin Pathol 135(1):54–61
Niitsu N et al (2011) A study on nm23-H1 expression in diffuse large B-cell lymphoma that was treated with CyclOBEAP plus rituximab therapy. Ann Hematol 90(2):185–192
Green TM et al (2012) Immunohistochemical double-hit score is a strong predictor of outcome in patients with diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol 30(28):3460–3467
Johnson NA et al (2012) Concurrent expression of MYC and BCL2 in diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol 30(28):3452–3459
Hu S et al (2013) MYC/BCL2 protein coexpression contributes to the inferior survival of activated B-cell subtype of diffuse large B-cell lymphoma and demonstrates high-risk gene expression signatures: a report from The International DLBCL Rituximab-CHOP Consortium Program. Blood 121(20): 4021–31; quiz 4250
Staiger AM et al (2017) Clinical impact of the cell-of-origin classification and the MYC/BCL2 dual expresser status in diffuse large B-cell lymphoma treated within prospective clinical trials of the German High-Grade Non-Hodgkin’s Lymphoma Study Group. J Clin Oncol 35(22):2515–2526
Choi WW et al (2009) A new immunostain algorithm classifies diffuse large B-cell lymphoma into molecular subtypes with high accuracy. Clin Cancer Res 15(17):5494–5502
Muris JJ et al (2006) Immunohistochemical profiling based on Bcl-2, CD10 and MUM1 expression improves risk stratification in patients with primary nodal diffuse large B cell lymphoma. J Pathol 208(5):714–723
Nyman H et al (2007) Prognostic impact of immunohistochemically defined germinal center phenotype in diffuse large B-cell lymphoma patients treated with immunochemotherapy. Blood 109(11):4930–4935
Natkunam Y et al (2008) LMO2 protein expression predicts survival in patients with diffuse large B-cell lymphoma treated with anthracycline-based chemotherapy with and without rituximab. J Clin Oncol 26(3):447–454
Meyer PN et al (2011) Immunohistochemical methods for predicting cell of origin and survival in patients with diffuse large B-cell lymphoma treated with rituximab. J Clin Oncol 29(2):200–207
Hawkes EA, Barraclough A, Sehn LH (2022) Limited-stage diffuse large B-cell lymphoma. Blood 139(6):822–834
Chaganti S et al (2016) Guidelines for the management of diffuse large B-cell lymphoma. Br J Haematol 174(1):43–56
Sehn LH, Salles G (2021) Diffuse Large B-Cell Lymphoma. Reply N Engl J Med 384(23):2262
Chiodin G et al (2021) Insertion of atypical glycans into the tumor antigen-binding site identifies DLBCLs with distinct origin and behavior. Blood 138(17):1570–1582
Rovida A et al (2021) Exploiting B-cell Receptor Stereotypy to Design Tailored Immunotherapy in Chronic Lymphocytic Leukemia. Clin Cancer Res 27(3):729–739
Wang L et al (2022) Primary cutaneous peripheral T-cell lymphomas with a T follicular helper phenotype: An integrative clinical, pathological, and molecular case series study. Br J Dermatol
Koo M et al (2022) Human Germinal Center-associated Lymphoma (HGAL) Is a Reliable Marker of Normal and Neoplastic Follicular Helper T Cells Including Angioimmunoblastic T-Cell Lymphoma. Am J Surg Pathol 46(5):643–654
Loeffler-Wirth H et al (2022) Classifying germinal center derived lymphomas—Navigate a complex transcriptional landscape. Cancers (Basel) 14(14)
Pindzola GM et al (2022) Aberrant expansion of spontaneous splenic germinal centers induced by hallmark genetic lesions of aggressive lymphoma. Blood
Amanda S et al (2022) IRF4 drives clonal evolution and lineage choice in a zebrafish model of T-cell lymphoma. Nat Commun 13(1):2420
Frauenfeld L et al (2022) Diffuse large B-cell lymphomas in adults with aberrant coexpression of CD10, BCL6, and MUM1 are enriched in IRF4 rearrangements. Blood Adv 6(7):2361–2372
Ricker E et al (2020) Selective dysregulation of ROCK2 activity promotes aberrant transcriptional networks in ABC diffuse large B-cell lymphoma. Sci Rep 10(1):13094
Horn H et al (2015) Different biological risk factors in young poor-prognosis and elderly patients with diffuse large B-cell lymphoma. Leukemia 29(7):1564–1570
Khanlari M et al (2022) Blastoid high-grade B-cell lymphoma initially presenting in bone marrow: a diagnostic challenge. Mod Pathol 35(3):419–426
Fang H et al (2022) Reactive Intralymphovascular Immunoblastic Proliferations Mimicking Aggressive Lymphomas. Am J Surg Pathol 46(3):326–335
Minakata D et al (2018) A leukemic double-hit follicular lymphoma associated with a complex variant translocation, t(8;14;18)(q24;q32;q21), involving BCL2, MYC, and IGH. Cancer Genet 220:44–48
Tan DE et al (2013) Genome-wide association study of B cell non-Hodgkin lymphoma identifies 3q27 as a susceptibility locus in the Chinese population. Nat Genet 45(7):804–807
Strazza M et al (2021) PD-1-induced proliferating T cells exhibit a distinct transcriptional signature. Immunology 164(3):555–568
Li J et al (2021) Identification BCL6 and miR-30 family associating with Ibrutinib resistance in activated B-cell-like diffuse large B-cell lymphoma. Med Oncol 38(4):33
Gonzalez-Figueroa P et al (2021) Follicular regulatory T cells produce neuritin to regulate B cells. Cell 184(7):1775-1789 e19
Jo T et al (2020) LUBAC accelerates B-cell lymphomagenesis by conferring resistance to genotoxic stress on B cells. Blood 136(6):684–697
Bucher P et al (2020) Targeting chronic NFAT activation with calcineurin inhibitors in diffuse large B-cell lymphoma. Blood 135(2):121–132
Yang Y et al (2016) Targeting non-proteolytic protein ubiquitination for the treatment of diffuse large B cell lymphoma. Cancer Cell 29(4):494–507
Duns G et al (2021) Characterization of DLBCL with a PMBL gene expression signature. Blood 138(2):136–148
Watanabe T (2021) The tumor microenvironment in follicular lymphoma: its pro-malignancy role with therapeutic potential. Int J Mol Sci 22(10)
Cheson BD et al (2014) Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol 32(27):3059–3068
El-Galaly TC et al (2018) FDG-PET/CT in the management of lymphomas: current status and future directions. J Intern Med 284(4):358–376
Adams HJ et al (2014) FDG PET/CT for the detection of bone marrow involvement in diffuse large B-cell lymphoma: systematic review and meta-analysis. Eur J Nucl Med Mol Imaging 41(3):565–574
Persky DO et al (2020) Positron emission tomography–directed therapy for patients with limited-stage diffuse large B-cell lymphoma: results of intergroup national clinical trials network study S1001. J Clin Oncol 38(26):3003–3011
Lamy T et al (2018) R-CHOP 14 with or without radiotherapy in nonbulky limited-stage diffuse large B-cell lymphoma. Blood 131(2):174–181
Persky DO et al (2008) Phase II study of rituximab plus three cycles of CHOP and involved-field radiotherapy for patients with limited-stage aggressive B-cell lymphoma: Southwest Oncology Group study 0014. J Clin Oncol 26(14):2258–2263
Roschewski M, Phelan JD, Jaffe ES (2024) Primary large b-cell lymphomas of immune-privileged sites. Blood 14(3):345–357
Pfreundschuh M et al (2011) CHOP-like chemotherapy with or without rituximab in young patients with good-prognosis diffuse large-B-cell lymphoma: 6-year results of an open-label randomised study of the MabThera International Trial (MInT) Group. Lancet Oncol 12(11):1013–1022
Shahbazi S, Peer CJ, Figg WD (2014) Prolonged low intensity EPOCH-rituximab has improved toxicity in Burkitt lymphoma compared with standard short, high intensity therapy. Cancer Biol Ther 15(9):1117–1119
Odejide OO et al (2015) Limited stage diffuse large B-cell lymphoma: comparative effectiveness of treatment strategies in a large cohort of elderly patients. Leuk Lymphoma 56(3):716–724
Kwon J et al (2015) Additional survival benefit of involved-lesion radiation therapy after R-CHOP chemotherapy in limited stage diffuse large B-cell lymphoma. Int J Radiat Oncol Biol Phys 92(1):91–98
Wiernik PH et al (2008) Lenalidomide monotherapy in relapsed or refractory aggressive non-Hodgkin’s lymphoma. J Clin Oncol 26(30):4952–4957
Nowakowski GS et al (2015) Lenalidomide combined with R-CHOP overcomes negative prognostic impact of non-germinal center B-cell phenotype in newly diagnosed diffuse large B-Cell lymphoma: a phase II study. J Clin Oncol 33(3):251–257
Vitolo U et al (2014) Lenalidomide plus R-CHOP21 in elderly patients with untreated diffuse large B-cell lymphoma: results of the REAL07 open-label, multicentre, phase 2 trial. Lancet Oncol 15(7):730–737
Nowakowski GS et al (2021) Addition of lenalidomide to R-CHOP improves outcomes in newly diagnosed diffuse large B-cell lymphoma in a randomized phase II US intergroup study ECOG-ACRIN E1412. J Clin Oncol 39(12):1329–1338
Nowakowski GS et al (2021) ROBUST: a phase III study of lenalidomide plus R-CHOP versus placebo plus R-CHOP in previously untreated patients with ABC-type diffuse large B-cell lymphoma. J Clin Oncol 39(12):1317–1328
Leonard JP et al (2017) Randomized Phase II Study of R-CHOP With or Without Bortezomib in Previously Untreated Patients With Non-Germinal Center B-Cell-Like Diffuse Large B-Cell Lymphoma. J Clin Oncol 35(31):3538–3546
Wilson WH et al (2015) Targeting B cell receptor signaling with ibrutinib in diffuse large B cell lymphoma. Nat Med 21(8):922–926
Elstrom RL et al (2008) Combined PET and low-dose, noncontrast CT scanning obviates the need for additional diagnostic contrast-enhanced CT scans in patients undergoing staging or restaging for lymphoma. Ann Oncol 19(10):1770–1773
Cheah CY et al (2013) Limited role for surveillance PET-CT scanning in patients with diffuse large B-cell lymphoma in complete metabolic remission following primary therapy. Br J Cancer 109(2):312–317
Cheung MC et al (2018) Are We Choosing Wisely in Lymphoma? Excessive Use of Surveillance CT Imaging in Patients With Diffuse Large B-cell Lymphoma (DLBCL) in Long-term Remission. Clin Lymphoma Myeloma Leuk 18(1):e27–e34
Sehn LH (2012) Chemotherapy alone for localized diffuse large B-cell lymphoma. Cancer J 18(5):421–426
Devic S et al (2010) Defining radiotherapy target volumes using 18F-fluoro-deoxy-glucose positron emission tomography/computed tomography: still a Pandora’s box? Int J Radiat Oncol Biol Phys 78(5):1555–1562
Andre MPE et al (2017) Early positron emission tomography response–adapted treatment in stage I and II Hodgkin lymphoma: final results of the randomized EORTC/LYSA/FIL H10 trial. J Clin Oncol 35(16):1786–1794
Mikhaeel NG et al (2016) Combination of baseline metabolic tumour volume and early response on PET/CT improves progression-free survival prediction in DLBCL. Eur J Nucl Med Mol Imaging 43(7):1209–1219
Zhao P, Yu T, Pan Z (2021) Prognostic value of the baseline 18F-FDG PET/CT metabolic tumour volume (MTV) and further stratification in low-intermediate (L-I) and high-intermediate (H-I) risk NCCNIPI subgroup by MTV in DLBCL MTV predict prognosis in DLBCL. Ann Nucl Med 35(1):24–30
Wilson WH et al (2021) Effect of ibrutinib with R-CHOP chemotherapy in genetic subtypes of DLBCL. Cancer Cell 39(12):1643-1653 e3
Gisselbrecht C et al (2010) Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol 28(27):4184–4190
Gonzalez Barca E (2023) Developing new strategies for relapsed/refractory diffuse large B-Cell lymphoma. J Clin Med 12(23):173–187
Crump M et al (2017) Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood 130(16):1800–1808
Chavez JC, Bachmeier C, Kharfan-Dabaja MA (2019) CAR T-cell therapy for B-cell lymphomas: clinical trial results of available products. Ther Adv Hematol 10:2040620719841581
Sermer D et al (2020) Outcomes in patients with DLBCL treated with commercial CAR T cells compared with alternate therapies. Blood Adv 4(19):4669–4678
Kochenderfer JN et al (2017) Long-duration complete remissions of diffuse large B cell lymphoma after anti-CD19 chimeric antigen receptor T cell therapy. Mol Ther 25(10):2245–2253
Siddiqi T et al (2023) Lisocabtagene maraleucel in chronic lymphocytic leukaemia and small lymphocytic lymphoma (TRANSCEND CLL 004): a multicentre, open-label, single-arm, phase 1–2 study. Lancet 402(10402):641–654
Abramson JS et al (2020) Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet 396(10254):839–852
Schuster SJ et al (2019) Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med 380(1):45–56
Neelapu SS et al (2017) Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med 377(26):2531–2544
Westin J, Sehn LH (2022) CAR T cells as a second-line therapy for large B-cell lymphoma: a paradigm shift? Blood 139(18):2737–2746
Kuhnl A et al (2022) A national service for delivering CD19 CAR-Tin large B-cell lymphoma - The UK real-world experience. Br J Haematol 198(3):492–502
Al-Mashhadi AL et al (2024) Real-world outcomes following third or subsequent lines of therapy: A Danish population-based study on 189 patients with relapsed/refractory large B-cell lymphomas. Br J Haematol 204(3):839–848
Locke FL et al (2019) Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1–2 trial. Lancet Oncol 20(1):31–42
Lin JK et al (2019) Cost effectiveness of chimeric antigen receptor T-cell therapy in multiply relapsed or refractory adult large B-cell lymphoma. J Clin Oncol 37(24):2105–2119
Czuczman MS et al (2017) A phase 2/3 multicenter, randomized, open-label study to compare the efficacy and safety of lenalidomide versus investigator’s choice in patients with relapsed or refractory diffuse large B-cell lymphoma. Clin Cancer Res 23(15):4127–4137
Davids MS et al (2017) Phase I first-in-human study of venetoclax in patients with relapsed or refractory non-Hodgkin lymphoma. J Clin Oncol 35(8):826–833
Zauderer MG et al (2022) EZH2 inhibitor tazemetostat in patients with relapsed or refractory, BAP1-inactivated malignant pleural mesothelioma: a multicentre, open-label, phase 2 study. Lancet Oncol 23(6):758–767
Harrington F et al (2021) Genomic characterisation of diffuse large B-cell lymphoma. Pathology 53(3):367–376
Tilly H et al (2022) Polatuzumab vedotin in previously untreated diffuse large B-cell lymphoma. N Engl J Med 386(4):351–363
Lee A (2021) Loncastuximab tesirine: first approval. Drugs 81(10):1229–1233
Caimi PF et al (2021) Loncastuximab tesirine in relapsed or refractory diffuse large B-cell lymphoma (LOTIS-2): a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol 22(6):790–800
Kalakonda N et al (2020) Selinexor in patients with relapsed or refractory diffuse large B-cell lymphoma (SADAL): a single-arm, multinational, multicentre, open-label, phase 2 trial. Lancet Haematol 7(7):e511–e522
Zinzani PL et al (2021) RE-MIND: comparing tafasitamab+ lenalidomide (L-MIND) with a real-world lenalidomide monotherapy cohort in relapsed or refractory diffuse large B-cell lymphoma. Clin Cancer Res 27(22):6124–6134
Locke FL et al (2022) Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N Engl J Med 386(7):640–654
Budde LE et al (2022) Safety and efficacy of mosunetuzumab, a bispecific antibody, in patients with relapsed or refractory follicular lymphoma: a single-arm, multicentre, phase 2 study. Lancet Oncol 23(8):1055–1065
Hutchings M et al (2021) Glofitamab, a novel, bivalent CD20-targeting T-cell–engaging bispecific antibody, induces durable complete remissions in relapsed or refractory B-cell lymphoma: a phase I trial. J Clin Oncol 39(18):1959–1970
Hutchings M et al (2021) Dose escalation of subcutaneous epcoritamab in patients with relapsed or refractory B-cell non-Hodgkin lymphoma: an open-label, phase 1/2 study. Lancet 398(10306):1157–1169
Hodi FS et al (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363(8):711–723
Robert C et al (2011) Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 364(26):2517–2526
Sharpe AH, Pauken KE (2018) The diverse functions of the PD1 inhibitory pathway. Nat Rev Immunol 18(3):153–167
Wu X et al (2019) Application of PD-1 Blockade in Cancer Immunotherapy. Comput Struct Biotechnol J 17:661–674
Acknowledgements
We thank our laboratory members for their helpful discussions and we apologize to the colleagues whose work could not be cited due to space constraints.
Authors’ contributions
Yuanfei Shi and Yi Xu wrote the manuscript. Huafei Shen collected the related literature. Hongyan Tong, Jie Jin, Dawei Cui and Wanzhuo Xie participated in the design of the review and revised the manuscript. All authors have read and approved the final manuscript.
Funding
This work was supported in part by the Research Plan of the NationalNatural Science Foundation of China (No.8230010226).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This review was reviewed and approved by Department of Hematology, The First Affiliated Hospital, College of Medicine, Zhejiang University.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shi, Y., Xu, Y., Shen, H. et al. Advances in biology, diagnosis and treatment of DLBCL. Ann Hematol (2024). https://doi.org/10.1007/s00277-024-05880-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00277-024-05880-z