Abstract
Background
Immune thrombocytopenia (ITP) is an immune-mediated disease that results in low platelet counts. Despite appropriate treatment, many patients continue to experience refractory disease. Fostamatinib, an oral spleen tyrosine kinase (SYK) inhibitor, has emerged as a promising option for refractory ITP.
Objective
This meta-analysis aims to evaluate the efficacy and safety of fostamatinib compared to conventional therapy in adults aged ≥ 18 years with refractory ITP.
Materials and methods
Literature search was conducted in PubMed, Scopus, Embase, and clinicaltrials.gov databases from inception to March 31, 2024. Randomized controlled trials (RCTs) assessing the safety and efficacy of fostamatinib in adults with refractory ITP were included. Data extraction, risk of bias assessment, and statistical analysis were performed following PRISMA guideline.
Results
A total of 495 articles were screened, with three RCTs meeting the inclusion criteria. Fostamatinib therapy demonstrated superior efficacy in achieving stable platelet response by week 24 (ORR 0.80; 95%CI 0.72–0.88), platelet count ≥ 50,000/µL at weeks 12 (ORR 0.80; 95%CI 0.72–0.90) and week 24 (ORR 0.82; 95%CI 0.72–0.90). Additionally, fostamatinib improves platelet counts in subjects with a baseline count of < 15,000/µL. The Number Needed to Treat (NNT) was calculated as 10. Adverse effects include diarrhea (RR 2.32; 95%CI 1.11–4.84), hypertension (RR 2.33; 95%CI 1.00-5.43), and abnormal liver function tests (RR 4.18; 95% CI 1.00-17.48). Interestingly, the occurrences of nausea (RR 1.77; 95% CI 0.33–9.67) and rash (RR 2.28; 95% CI 0.50-10.29) did not achieve statistical significance.
Conclusion
This meta-analysis provides robust evidence supporting the efficacy of fostamatinib in improving platelet counts and achieving therapeutic goals in adults with refractory ITP. However, fostamatinib’s safety profile warrants consideration due to higher rates of diarrhea, hypertension, and abnormal liver function tests.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Immune thrombocytopenia (ITP) is an autoimmune disorder characterized by a low platelet count, which can lead to an increased risk of bleeding and other complications [1]. The incidence of ITP was reported to be 3.3 adults per 100,000 per year [2]. Corticosteroids serve as the first-line treatment for ITP [3]. Despite the availability of various treatment options, a significant proportion of adults with ITP experience refractory disease, wherein conventional therapies either fail to provide an adequate platelet response or are associated with intolerable side effects.
Spleen tyrosine kinase (SYK) is expressed primarily in hematopoietic cells and plays a key role in the signaling of activating Fc receptor (FcR) and the B-cell receptor (BCR) [4]. Fostamatinib (formerly R788), an oral SYK inhibitor, was initially developed for rheumatoid arthritis treatment [5, 6]. By inhibiting phosphorylation of SYK substrate linker for activation of T cell and B cell, fostamatinib reduces antibody-mediated and immune-complex-mediated inflammation [7, 8]. Fostamatinib later received FDA approval in April 2018 as a promising therapeutic option for patients with refractory ITP [9]. In patients with ITP, the fostamatinib dose started at 100 mg twice daily and can be increased up to 150 mg twice daily [8]. Previous randomized controlled trials (RCTs) have assessed the efficacy and safety of fostamatinib compared to other treatments in this patient population [10, 11]. However, the results of these studies have shown variability, and adverse effects have not been statistically described. Thus, there is a need for a comprehensive synthesis of existing evidence to determine the overall efficacy and safety profile of fostamatinib in refractory ITP.
This meta-analysis aims to systematically review and analyze the available RCTs to evaluate the efficacy and safety of fostamatinib in combination with conventional therapy compared to conventional therapy alone in adults aged ≥ 18 years with refractory ITP. The primary outcomes of interest include stable platelet response by week 24, platelet count at week 12 and week 24, and achievement of platelet count goals in subjects with a baseline count < 15,000/µL.
By synthesizing the existing evidence, this meta-analysis aims to provide clinicians, researchers, and policymakers with valuable insights into the potential benefits and risks of fostamatinib in the management of refractory ITP, ultimately informing clinical decision-making and guiding future research efforts in this area.
Materials and methods
Objective
The aim of this meta-analysis was to evaluate the efficacy and safety of fostamatinib in combination with conventional therapy compared to conventional therapy alone in adults aged ≥ 18 years with refractory immune thrombocytopenia (ITP). The primary outcome in this study is efficacy measures by using the Objective Response Rate (ORR). The ORR was assessed based on stable platelet response by week 24, platelet count ≥ 50,000/µL at weeks 12 and 24, and achievement of a count ≥ 30,000/µL and at least 20,000/µL above baseline at weeks 12 and 24 in subjects with a baseline count < 15,000/µL. The secondary outcome measures focused on adverse events associated with fostamatinib treatment.
Search strategy
A comprehensive literature search was conducted using the following databases: PubMed (MEDLINE), Scopus, Embase, and the Registry of clinicaltrials.gov. The search strategy employed the keywords “Fostamatinib and Immune thrombocytopenia” or “Fostamatinib and ITP”. The literature search encompassed articles published from the inception of each database up to March 31, 2024. The search was restricted to articles published in English only. Search queries for each database can be found in the supplementary file. The authors adhered to PRISMA 2020 guideline [12].
Inclusion and exclusion criteria
Inclusion criteria: Studies that provided data on the safety and efficacy of fostamatinib in adults with refractory ITP.
Exclusion criteria: Review articles, case reports, case series, preclinical studies, and clinical studies irrelevant to fostamatinib or ITP. Studies without safety or efficacy outcomes were also excluded.
Study selection
Two authors (J.J. and T.K.) independently screened the titles and abstracts of the identified articles. Any discrepancies in the screening process were resolved by a third researcher (S.T.).
Data extraction
Data extraction was performed independently by two authors (N.B. and S.T.) using a standardized data extraction form. The extracted data included baseline characteristics, efficacy outcomes (stable platelet response by week 24, platelet count at week 12 and week 24, achievement of platelet count goals in subjects with baseline count < 15,000/µL), and adverse events. In instances where data were missing or unclear, we made assumptions based on available information and consulted with other experts in the field. The full data extraction form can be found in the supplementary file.
Risk of bias assessment
The risk of bias in the included studies was assessed using the Cochrane Risk of Bias Tool (ROB-II) [13] by two independent researchers (J.J. and S.T.). Any disagreements in the assessment were resolved through discussion or consultation with a third researcher (N.B.).
Statistical analysis
Data analysis was conducted using STATA version 18.0 software (StataCorp, College Station, TX, USA). The meta-analysis was performed to calculate pooled estimates of risk ratio, risk difference, and Number Needed to Treat (NNT) concerning both efficacy and adverse events associated with fostamatinib treatment. Heterogeneity analysis was studied, and a fixed-effects model was utilized.
Result
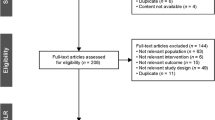
From the database, a total of 495 articles were identified: 91 from PubMed, 138 from Scopus, 256 from Embase, and 10 from clinicaltrials.gov. After careful screening of the articles, three randomized controlled trials (N = 123) were included (Fig. 1).
The risk of bias was low in all studies. All studies provided detailed information about the allocation sequences, which were stratified random. Two studies balanced patients with prior splenectomy and the degree of thrombocytopenia, while one study stratified by baseline platelet count (< or ≥ 15,000/µL). Conflict arose in the allocation concealment part; while one author (J.J) rated all studies as “No information,” the other authors (S.T. and N.B) pointed out that in the context of a large trial run by an experienced clinical trials unit, responding with “Probably yes” rather than “No information” is more reasonable [13]. Our judgment differed from a previous meta-analysis, which rated it as some concern due to no information [14, 15]. No baseline differences between treatment arms were observed. The comprehensive risk of bias assessment is summarized (Supplementary Fig. 1).
Baseline characteristics
The main characteristics of patients included in the studies are shown (Table 1). In total, these studies encompassed 184 patients with refractory ITP. Of these participants, 123 were administered fostamatinib in conjunction with conventional therapy, while 61 patients received conventional therapy alone. The dose of fostamatinib varied across the studies, with patients receiving either 100 mg PO bid per day or 150 mg PO bid per day. All patients enrolled in the studies had previously undergone 2 to 3 lines of therapy for ITP before participating in the trials. The majority of these prior treatments consisted of corticosteroids, which were utilized by over 90% of the patients. Additionally, more than 60% of the patients had previously been treated with thrombopoietin agents or immunosuppressive drugs. Upon comparing the baseline characteristics between the fostamatinib group and the conventional therapy group, no significant differences were observed. The demographic details of the patients, such as age and gender distribution were well-balanced between the two treatment groups.
Conventional therapy
In the three randomized controlled trials (RCTs) included in our meta-analysis, the conventional therapies utilized in these studies were notably similar, contributing to low heterogeneity between the studies.
Two of the studies primarily consisted of a single medication such as corticosteroids at a dosage of < 20 mg prednisolone equivalent per day, azathioprine, or danazol throughout the study duration. Similarly, the third study permitted the use of corticosteroids at a dosage of ≤ 10 mg prednisolone per day, azathioprine, or danazol throughout the study period.
The uniformity in the conventional therapy regimens across the three studies is notable. This similarity minimizes the potential for heterogeneity in treatment effects, thereby enhancing the comparability of the study outcomes. By employing consistent treatment protocols, the studies aimed to provide a standardized control group against which the efficacy and safety of fostamatinib could be evaluated.
The adequacy of the conventional therapy dosage in the control arm of the included studies depends on several factors, including the severity of the patient’s condition, previous treatment responses, and potential side effects. While the specified corticosteroid dosage (< 20 mg prednisolone equivalent per day) is within the range commonly used in clinical practice for ITP management, the dosage threshold for azathioprine and danazol remains unspecified.
Primary outcome measures
The primary outcome in this study is efficacy measures by using the Objective Response Rate (ORR). The ORR for stable platelet response by week 24 was significantly higher in fostamatinib therapy group compared to conventional therapy alone, with an ORR of 0.80 (95%CI 0.72 to 0.88, I2 = 18.5%) (Fig. 2), favoring fostamatinib group. Heterogeneity analysis indicated a very low level of heterogeneity between studies (I2 = 18.5%). The pooled risk difference was − 0.10 (95%CI -0.14 to -0.06, I2 = 78.4%) and the Number Needed to Treat (NNT) was 10.
The outcome of platelet Count ≥ 50,000/µL at week 12 ORR was 0.80 (95%CI 0.72 to 0.90, I2 = 0%) (Fig. 3). The outcome of platelet Count ≥ 50,000/µL at week 24 ORR was 0.82 (95%CI 0.72 to 0.90, I2 = 33.8%).
Among subjects with a baseline platelet count < 15,000/µL, achievement of a count ≥ 30,000/µL and at least 20,000/µL above baseline at week 12 ORR was 0.81 (95%CI 0.69 to 0.95, I2 = 0%), while achievement of a count ≥ 30,000/µL and at least 20,000/µL above baseline at week 24 ORR was 0.82 (95%CI 0.73 to 0.93, I2 = 0%) (Supplementary Figs. 2 and 3).
Secondary outcome measure
Secondary outcome included adverse events. The result indicated that some AEs were significantly higher in fostamatinib group than conventional therapy such as diarrhea (RR = 2.32, RD = 0.23; 95%CI 0.02 to 0.44), hypertension (RR = 2.33, RD = 0.17; 95%CI 0.01 to 0.32), and liver function abnormality (RR = 4.18, RD = 0.12; 95%CI 0.02 to 0.22) (Fig. 4). Among side effects of any grade, nausea (RR = 1.77, RD = 0.08; 95%CI -0.09 to 0.26) and rash (RR = 2.28, RD = 0.06; 95%CI -0.01 to 0.12) were not statistically significant (Supplementary Figs. 4 and 5).
Discussion
This meta-analysis, which examined the efficacy and side effects of fostamatinib, included three randomized controlled trials with 184 patients encompassing refractory ITP. Our primary outcome measures, specifically the Objective Response Rate (ORR), demonstrated significantly high values across all measured outcomes, such as stable platelet response by week 24. The consistent and favorable response to fostamatinib was evident across different time points. Given that this study was conducted in a refractory population, the Number Needed to Treat (NNT) of 10 appears to be a reasonable metric for clinical use. These findings reinforce the potential of fostamatinib as an effective therapeutic option for refractory ITP, aligning with previous studies that emphasized its role in improving platelet counts and achieving therapeutic goals.
In patients with ITP, head-to-head studies comparing treatments, mostly in persistent/chronic condition, are limited and a network meta-analysis approach is utilized to help clinicians decide between treatment options. We summarized the network meta-analysis in both newly diagnosed and chronic ITP (Tables 2 and 3, and 4). Treatment options for ITP vary in disease progression, effectiveness, and safety profiles. In primary ITP among children, a study in 2020 indicated that using 2 g/kg of IVIG results in a better response rate with fewer adverse reactions compared to other monotherapy options [16].
For newly diagnosed ITP, combination therapies have shown significantly better outcomes than prednisolone or dexamethasone monotherapy in terms of overall and sustained response. However, 15.3% of combination therapies between rituximab and dexamethasone reported grade 3 or higher adverse events [18]. Therefore, alternative combination therapies such as dexamethasone plus human recombinant thrombopoietin (rhTPO), rituximab plus prednisolone, or eltrombopag plus rituximab, as well as rhTPO plus prednisolone, are suggested due to their significant early response and favorable clinical outcomes with a lower chance of adverse reactions [17, 18, 24].
For persistent or chronic ITP, previous studies suggested that thrombopoietin receptor agonists (TPO-RAs) demonstrate superior clinical responses, including longer duration of platelet response, higher overall response rates, lower rescue therapy use, and fewer bleeding events compared to other treatment options. However, avatrombopag has shown a better platelet response rate than TPO-RAs with lower bleeding risk and no significant differences in treatment-related adverse reactions when compared to eltrombopag and romiplostim. Hence, it is recommended as an alternative treatment [19,20,21,22,23].
In the utilization of fostamatinib, previous studies are consistent with our findings, demonstrating a statistically significant increase in durable platelet response and superiority when compared to rituximab [14]. Additionally, it has shown a lower incidence of bleeding and demonstrated high cumulative probabilities of being the best treatment [21]. However, a meta-analysis in 2022, which included 19 drug therapies, contrasted that fostamatinib could not demonstrate higher efficacy than placebo. This disparity might be due to the very high heterogeneity of the studies, which included data from both newly diagnosed and chronic ITP patient, as well as the multitude of interventions studied, diluting the effect of fostamatinib [24].
There is no question regarding fostamatinib’s efficacy in chronic ITP patients. However, the question arises whether it can be used as a second-line treatment or beyond. Post hoc analysis data in 2020 suggested that fostamatinib was more effective as a second-line (78%) than third-or-later-line (48%) therapy for ITP [25]. Nevertheless, it’s crucial to note that fostamatinib exhibits a slower response compared to TPO-RA treatments, which often produce an “early response” effect within 2 weeks. This slower response might prompt clinicians, in severe cases, to consider other treatment options without waiting for fostamatinib’s response.
In alignment with prior research, our analysis underscores the challenges in managing ITP, particularly the variable response to conventional therapies and the potential for adverse effects. By pooling data from previous randomized controlled trials, we enhance the power to detect differences in outcomes and provide a more precise estimate of treatment effects. We noted that our study has very low heterogeneity between studies (I2 = 18.5%).The results of this meta-analysis have important implications for the management of refractory ITP. Despite some significant adverse events, for instance, diarrhea, hypertension, and liver function abnormal, the severity was less than grade 3. These mild side effects are consistent with the real-world data reported in 2023 and 2024 [26, 27]. However, physicians should be cautious in patients who have pre-existing conditions such as cirrhosis, hypertension, or gastrointestinal disease.
Although our study revealed promising data, there are some limitations due to the limited number of studies and participants included in this meta-analysis. Future research should focus on evaluating the long-term efficacy and safety of fostamatinib, as well as exploring potential strategies to mitigate the identified adverse events.
Conclusion
Our meta-analysis underscores the potential of fostamatinib as a promising therapeutic option for adults with refractory immune thrombocytopenia (ITP). Fostamatinib, when combined with conventional therapy, demonstrated a significantly higher Objective Response Rate (ORR) in achieving stable platelet responses by week 24 and attaining desired platelet counts at both week 12 and 24 compared to conventional therapy alone. The consistent and favorable response observed across various time points emphasizes the drug’s efficacy in this challenging patient population.
Despite the promising efficacy, fostamatinib was associated with certain adverse events, including diarrhea, hypertension, and liver function abnormalities. However, the severity of these adverse events was generally mild (grade < 3), aligning with real-world data from recent years. Hence, while fostamatinib offers potential benefits, clinicians should be vigilant, particularly when considering patients with pre-existing conditions such as cirrhosis, hypertension, or gastrointestinal disease.
This meta-analysis provides valuable insights into the efficacy and safety profile of fostamatinib in refractory ITP and reinforces its potential as a viable treatment option. Nevertheless, the limited number of studies and participants in our analysis highlight the need for more extensive research to further validate these findings and explore strategies to minimize the identified adverse events. Future studies should also focus on assessing the long-term efficacy, safety, and optimal dosing strategies of fostamatinib to guide its appropriate use in the management of refractory ITP.
Registration
This study was registered in the International Prospective Register of Systematic Reviews (PROSPERO) web registry. The registration number for this review is CRD42024528563.
Data availability
No datasets were generated or analysed during the current study.
References
Rodeghiero F, Stasi R, Gernsheimer T et al (2009) Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood 113(11):2386–2393. https://doi.org/10.1182/blood-2008-07-162503
Terrell DR, Beebe LA, Vesely SK, Neas BR, Segal JB, George JN (2010) The incidence of immune thrombocytopenic purpura in children and adults: a critical review of published reports. Am J Hematol 85(3):174–180. https://doi.org/10.1002/ajh.21616
Neunert C, Terrell DR, Arnold DM et al (2019) American Society of Hematology 2019 guidelines for immune thrombocytopenia. Blood Adv 3(23):3829–3866. https://doi.org/10.1182/bloodadvances.2019000966
Cooper N, Ghanima W, Hill QA, Nicolson PL, Markovtsov V, Kessler C (2023) Recent advances in understanding spleen tyrosine kinase (SYK) in human biology and disease, with a focus on fostamatinib. Platelets 34(1):2131751. https://doi.org/10.1080/09537104.2022.2131751
Weinblatt ME, Kavanaugh A, Burgos-Vargas R et al (2008) Treatment of rheumatoid arthritis with a syk kinase inhibitor: a twelve-week, randomized, placebo-controlled trial. Arthritis Rheum 58(11):3309–3318. https://doi.org/10.1002/art.23992
Weinblatt ME, Kavanaugh A, Genovese MC, Musser TK, Grossbard EB, Magilavy DB (2010) An oral spleen tyrosine kinase (syk) inhibitor for rheumatoid arthritis. N Engl J Med 363(14):1303–1312. https://doi.org/10.1056/NEJMoa1000500
Braselmann S, Taylor V, Zhao H et al (2006) R406, an orally available spleen tyrosine kinase inhibitor blocks fc receptor signaling and reduces immune complex-mediated inflammation. J Pharmacol Exp Ther 319(3):998–1008. https://doi.org/10.1124/jpet.106.109058
Matsukane R, Suetsugu K, Hirota T, Ieiri I (2022) Clinical pharmacokinetics and Pharmacodynamics of Fostamatinib and its active Moiety R406. Clin Pharmacokinet 61(7):955–972. https://doi.org/10.1007/s40262-022-01135-0
Kirin KH (2018) mAb X-linked F. FDA new drug approvals in Q2 2018. Nat Reviews| Drug Discovery 17:537
Bussel JB, Arnold DM, Boxer MA et al (2019) Long-term fostamatinib treatment of adults with immune thrombocytopenia during the phase 3 clinical trial program. Am J Hematol 94(5):546–553. https://doi.org/10.1002/ajh.25444
Kuwana M, Ito T, Kowata S et al (2023) Fostamatinib for the treatment of Japanese patients with primary immune thrombocytopenia: a phase 3, placebo-controlled, double-blind, parallel-group study. Br J Haematol 200(6):802–811. https://doi.org/10.1111/bjh.18582
Page MJ, McKenzie JE, Bossuyt PM et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. https://doi.org/10.1136/bmj.n71
Sterne JAC, Savovic J, Page MJ et al (2019) RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 366:l4898. https://doi.org/10.1136/bmj.l4898
Yang R, Lin L, Yao H, Ji O, Shen Q (2019) Therapeutic options for adult patients with previously treated immune thrombocytopenia - a systematic review and network meta-analysis. Hematology 24(1):290–299. https://doi.org/10.1080/16078454.2019.1568659
Cohen I, Goldvaser H, Kirgner I et al (2021) Targeted therapies for immune thrombocytopenic purpura: a meta-analysis of randomized controlled trials. Ann Hematol 100(12):2879–2887. https://doi.org/10.1007/s00277-021-04669-8
Acero-Garces DO, Garcia-Perdomo HA (2020) First Line treatments for newly diagnosed primary Immune Thrombocytopenia in children: a systematic review and network Meta-analysis. Curr Pediatr Rev 16(1):61–70. https://doi.org/10.2174/1573396315666191023122542
Arai Y, Jo T, Matsui H, Kondo T, Takaori-Kondo A (2018) Comparison of up-front treatments for newly diagnosed immune thrombocytopenia -a systematic review and network meta-analysis. Haematologica 103(1):163–171. https://doi.org/10.3324/haematol.2017.174615
Wang Y, Sheng L, Han F et al (2023) Efficacy and safety of treatments in newly diagnosed adult primary immune thrombocytopenia: a systematic review and network meta-analysis. EClinicalMedicine 56:101777. https://doi.org/10.1016/j.eclinm.2022.101777
Arai Y, Matsui H, Jo T, Kondo T, Takaori-Kondo A (2019) Comparison of treatments for persistent/chronic immune thrombocytopenia: a systematic review and network meta-analysis. Platelets 30(8):946–956. https://doi.org/10.1080/09537104.2018.1543864
Puavilai T, Thadanipon K, Rattanasiri S et al (2020) Treatment efficacy for adult persistent immune thrombocytopenia: a systematic review and network meta-analysis. Br J Haematol 188(3):450–459. https://doi.org/10.1111/bjh.16161
Wojciechowski P, Wilson K, Nazir J et al (2021) Efficacy and safety of Avatrombopag in patients with chronic Immune Thrombocytopenia: a systematic literature review and network Meta-analysis. Adv Ther 38(6):3113–3128. https://doi.org/10.1007/s12325-021-01752-4
Liu Y, Zhang HX, Su J, Geng QC, Lin X, Feng CX (2023) Efficacy and incidence of treatment-related adverse events of Thrombopoietin receptor agonists in adults with Immune Thrombocytopenia: a systematic review and network Meta-analysis of Randomized Controlled Study. Acta Haematol 146(3):173–184. https://doi.org/10.1159/000528642
Li T, Liu Q, Pu T, Liu J, Zhang A (2023) Efficacy and safety of thrombopoietin receptor agonists in children and adults with persistent and chronic immune thrombocytopenia: a meta-analysis. Expert Opin Pharmacother 24(6):763–774. https://doi.org/10.1080/14656566.2023.2198089
Zhou H, Fan J, He J, Hu S (2022) Comparative efficacy of 19 drug therapies for patients with idiopathic thrombocytopenic purpura: a multiple-treatments network meta-analysis. Ann Hematol 101(5):953–961. https://doi.org/10.1007/s00277-022-04784-0
Boccia R, Cooper N, Ghanima W et al (2020) Fostamatinib is an effective second-line therapy in patients with immune thrombocytopenia. Br J Haematol 190(6):933–938. https://doi.org/10.1111/bjh.16959
Jimenez-Barcenas R, Garcia-Donas-Gabaldon G, Campos-Alvarez RM et al (2024) Treatment with fostamatinib in patients with immune thrombocytopenia: experience from the andalusian region in Spain-the Fostasur Study. Br J Haematol. https://doi.org/10.1111/bjh.19443
Dranitsaris G, Peevyhouse A, Wood T, Kreychman Y, Neuhalfen H, Moezi M (2023) Fostamatinib or Thrombopoietic receptor agonists for the treatment of Chronic Immune Thrombocytopenia in Adult patients: a Real-World Assessment of Safety, effectiveness and cost. Acta Haematol. https://doi.org/10.1159/000533175
Funding
No funding was received.
Author information
Authors and Affiliations
Contributions
S.T. wrote the first manuscript, conceptualization, and data analysis. T.K. was involved in database search, screening of abstracts and full texts, and editing manuscript. N.B. involved in appraisal, data analysis, and editing manuscript. J.J. involved in database search and editing manuscript. S.S. was involved in database search and reviseing manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
None to declare.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tungjitviboonkun, S., Bumrungratanayos, N., Jitwimungsanon, J. et al. Efficacy and safety of fostamatinib in refractory immune thrombocytopenia: a meta-analysis from randomized controlled trials. Ann Hematol (2024). https://doi.org/10.1007/s00277-024-05824-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00277-024-05824-7