Abstract
Stenotrophomonas maltophilia (SM) bloodstream infections (BSIs) contribute to significant mortality in hematologic malignancy (HM) and hematopoietic stem cell transplantation (HSCT) patients. A risk score to predict SM BSI could reduce time to appropriate antimicrobial therapy (TTAT) and improve patient outcomes. A single center cohort study of hospitalized adults with HM/HSCT was conducted. Patients had ≥ 1 blood culture with a Gram-negative (GN) organism. A StenoSCORE was calculated for each patient. The StenoSCORE2 was developed using risk factors for SM BSI identified via logistic regression. Receiver operating characteristic (ROC) curves were plotted. Sensitivity and specificity for the StenoSCORE and StenoSCORE2 were calculated. Thirty-six SM patients and 534 non-SM patients were assessed. A StenoSCORE ≥ 33 points was 80% sensitive, 68% specific, and accurately classified 69% of GN BSIs. StenoSCORE2 variables included acute leukemia, prolonged neutropenia, mucositis, ICU admission, recent meropenem and/or cefepime exposure. The StenoSCORE2 performed better than the StenoSCORE (ROC AUC 0.84 vs. 0.77). A StenoSCORE2 ≥ 4 points was 86% sensitive, 76% specific, and accurately classified 77% of GN BSIs. TTAT was significantly longer for patients with SM BSI compared with non-SM BSI (45.16 h vs. 0.57 h; p < 0.0001). In-hospital and 28-day mortality were significantly higher for patients with SM BSI compared to non-SM BSI (58.3% vs. 18.5% and 66.7% vs. 26.4%; p-value < 0.0001). The StenoSCORE and StenoSCORE2 performed well in predicting SM BSIs in patients with HM/HSCT and GN BSI. Clinical studies evaluating whether StenoSCORE and/or StenoSCORE2 implementation improves TTAT and clinical outcomes are warranted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stenotrophomonas maltophilia is a multidrug resistant, Gram-negative bacteria that has emerged as an opportunistic and life-threatening pathogen in immunosuppressed and critically ill patients [1, 2]. S. maltophilia bloodstream infections (BSI) are associated with high mortality rates ranging from 21 to 69% [3]. Patients with hematologic malignancy and hematopoietic stem cell transplant (HSCT) are at an increased risk for developing S. maltophilia BSI due to widespread use of central venous catheters (CVC), neutropenia, exposure to broad-spectrum antibiotic therapy, and prolonged hospital lengths of stay [1, 3]. Prompt initiation of treatment active against S. maltophilia poses a clinical challenge due to intrinsic resistance to commonly employed antimicrobials for Gram-negative infections, including most β-lactams and aminoglycosides [4]. Historically, trimethoprim-sulfamethoxazole, minocycline, and/or levofloxacin have been first-line treatment options for S. maltophilia infections, however; these agents are not commonly initiated as empiric therapy for Gram-negative BSIs due to the low overall prevalence of S. maltophilia and toxicities associated with their use (e.g., trimethoprim-sulfamethoxazole) [2, 4].
Currently available rapid molecular diagnostic tests have the capabilities to quickly identify S. maltophilia from blood cultures within hours, however, many healthcare institutions do not have access to this technology [5]. Among patients with hematologic malignancy/HSCT and Gram-negative BSI, the use of a risk score to identify patients with an increased likelihood of having S. maltophilia BSI could reduce time to appropriate antimicrobial therapy and thus reduce mortality. The StenoSCORE was developed in an observational cohort of patients with hematologic malignancy/HSCT and Gram-negative BSI to better identify patients at risk for S. maltophilia BSI [4]. The scoring tool utilizes five variables which have either previously been identified as risk factors for S. maltophilia BSI or were found to be significant risk factors within the cohort. Possible scores range from 0 to 77 points with scores ≥ 41 points correctly classifying > 80% of Gram-negative BSI observations as S. maltophilia infections within the study [4]. Although the StenoSCORE represents a pragmatic tool for identifying patients that may benefit from S. maltophilia active therapy in the setting of Gram-negative BSI, it has not been externally validated outside of the original study. We sought to determine the utility of the StenoSCORE in a cohort of hematologic malignancy/HSCT patients with documented Gram-negative BSI as well as to determine if alternative variables better predicted S. maltophilia BSI within this patient population.
Methods
Study design and patient population
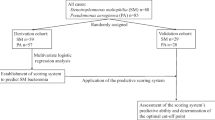
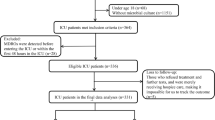
This was a single center, cohort study of adult patients with hematologic malignancy/HSCT admitted to Barnes Jewish Hospital between 5/1/2018 and 2/28/2023. Patients aged ≥ 18 years with hematologic malignancy actively receiving chemotherapy and/or received a HSCT within the preceding 12 months and had at least one blood culture positive for Enterobacterales or non-fermenting Gram-negative bacilli within the study period were included. A cohort of patients with S. maltophilia BSI was identified to serve as the primary case population of interest. For comparison, a second cohort of patients with BSI due to non-S. maltophilia Gram-negative organisms was subsequently identified to serve as controls. Only unique hospital encounters for BSIs were included in the study and patients could only be included into one of the two cohorts. Only the first episode of BSI meeting criteria was included for patients with multiple BSIs within the same hospital encounter.
Data collection and definitions
Patients were identified for study inclusion using ICD10 codes for hematologic malignancy, HSCT, and Gram-negative bacteremia. Relevant patient characteristics collected at the time of index culture included: malignancy diagnosis, comorbid conditions, vital signs, absolute neutrophil count (ANC), presence of mucositis, and indwelling CVC. Hospital and intensive care unit (ICU) admission and discharge data for both the current encounter and all hospital encounters in the preceding 3 months of index culture were collected. All microbiologic data in the 6 months preceding the index culture, antimicrobial therapy prescribed in the previous 3 months, and antimicrobial therapy for current BSI were reviewed.
BSI was defined as at least one blood culture positive for an included Gram-negative organism. Active chemotherapy was defined as receipt of a parenteral chemotherapeutic agent within the month preceding the index culture. Presence of mucositis was ascertained via chart review for oral ulcers noted within the patient’s active problem list or on physical exam. Consistent with the previous publication, neutropenia was defined as an ANC < 1500 cells/mm3 [4]. Appropriate antimicrobial therapy was defined as receipt of an appropriately dosed antimicrobial deemed susceptible based on the index culture antimicrobial susceptibility report determined using disk diffusion per CLSI standards [6]. An isolate was considered multidrug resistant if the organism was carbapenem resistant, the organism was an extended-spectrum β-lactamase producer, or if the culture contained Pseudomonas aeruginosa, Acinetobacter baumannii, Achromobacter species, or Enterobacterales that was resistant to at least one drug in three or more of the following antibiotic categories: piperacillin-tazobactam, extended-spectrum cephalosporins, fluoroquinolones, aminoglycosides, or carbapenems [7]. If A. baumannii was cultured, the isolate was considered multidrug resistant if it was resistant to ampicillin-sulbactam [7]. An isolate was considered a carbapenem resistant organism if it was resistant to at least one carbapenem or produced a carbapenemase as determined by use of the Xpert Carba-R assay (Cepheid, Sunnyvale, CA, USA) [8].
Statistical analysis
The primary endpoints of interest were identification of variables associated with S. maltophilia BSI as compared with non-S. maltophilia Gram-negative BSI. Categorical data were compared using Chi-square or Fisher’s exact test as appropriate. Continuous data were compared using Mann-Whitney U test. To assess independent risk factors for in-hospital mortality, a post-hoc multivariable logistic regression was performed using variables with a p-value of < 0.05 on univariable analysis.
A StenoSCORE was calculated for each patient using predetermined variables and point assignments described in the previously mentioned study (Table 1) [4]. The receiver operating characteristic (ROC) curve was plotted and area under the curve (AUC) was calculated. Discrimination was evaluated using ROC AUC with its corresponding 95% confidence interval and calibration was assessed using the Hosmer-Lemeshow goodness-of-fit test. Sensitivity, specificity, positive predictive value, and negative predictive value of the StenoSCORE were calculated to evaluate for an optimal point cutoff for S. maltophilia BSI prediction.
To evaluate variables associated with S. maltophilia BSI in our patient population, univariable analysis was used to assess the unadjusted association between independent variables and S. maltophilia BSI. Using a significance level of < 0.05, six variables were selected for inclusion into the binary logistic regression which would then comprise the StenoSCORE2. Points assigned for each variable in the risk score were derived from scaling variables by the smallest model coefficient in the binary logistic regression and rounding the result to the nearest integer. The total StenoSCORE2 was calculated by summation of the points associated with each variable applicable to a given patient. The model was assessed by plotting the ROC curve and determining discrimination and calibration as previously described [4]. Sensitivity, specificity, positive predictive value, and negative predictive value of the StenoSCORE2 were calculated. Statistical analyses were performed using SPSS Statistics version 25 (IBM corp, Armonk, NY, USA).
Results
In total, 36 patients with S. maltophilia BSI and 534 patients with non-S. maltophilia Gram-negative BSI were identified for inclusion. Organisms included in the non-S. maltophilia Gram-negative BSI group are listed in Table S1. Baseline characteristics for the study population are listed in Table 2. Compared with non-S. maltophilia Gram-negative BSI patients, patients with S. maltophilia BSI were more likely to have acute myeloblastic or lymphoblastic leukemia (75% vs. 46.8%; p-value < 0.0001), experience neutropenia for ≥ 7 days prior to the index culture date (77.8% vs. 35.2%; p-value < 0.0001), have mucositis (36.1% vs. 13.1%; p-value < 0.0001), ICU admission within 12 h of index culture (44.4% vs. 24.5%; p-value 0.008), have a prior history of S. maltophilia colonization or infection in the preceding 6 months (13.9% vs. 0.2%; p-value < 0.0001), and have received ≥ 3 days of carbapenem therapy (63.9% vs. 17.4%; p-value < 0.0001), cefepime (63.9% vs. 27.3%; p-value < 0.0001), or piperacillin-tazobactam (8.3% vs. 1.3%; p-value < 0.0001) within the preceding 3 months. The time from hospitalization to index blood culture was significantly longer in the S. maltophilia group compared with the non-S. maltophilia Gram-negative BSI group (19 days vs. 10 days; p-value < 0.0001). Degree of neutropenia and presence of CVC did not differ between groups. Time from index culture to initiation of appropriate antimicrobial therapy was 0.57 and 45.16 h in the non-S. maltophilia BSI and S. maltophilia BSI groups, respectively (p-value < 0.0001). Both in-hospital and 28-day mortality were significantly higher in the S. maltophilia BSI group compared with the non-S. maltophilia BSI group (58.3% vs. 18.5%, p-value < 0.0001 and 66.7% vs. 26.4%; p-value < 0.0001). In an exploratory multivariable analysis, S. maltophilia BSI and time to appropriate antimicrobials greater than 60 min were both independent predictors of in-hospital mortality (Table 3).
When assessing StenoSCOREs between groups, patients with S. maltophilia BSI had significantly higher scores compared with patients with non-S. maltophilia BSI (42 vs. 23; p-value < 0.0001). ROC curve analysis of the StenoSCORE performance in our cohort produced an AUC 0.77 (95% CI 0.70–0.84) indicating acceptable discrimination (Fig. 1). Table 4 summarizes the sensitivity, specificity, and accuracy of the StenoSCORE at various point cutoffs. A StenoSCORE ≥ 33 had the highest combined sensitivity and specificity with values of 80% and 68%, respectively.
Receiver operator characteristic curves illustrating the sensitivity and specificity of the StenoSCORE and StenoSCORE2. The StenoSCORE exhibited acceptable discrimination with an AUC of 0.77 (95% CI 0.70-0.84) while the StenoSCORE2 exhibited excellent discrimination with an AUC of 0.84 (95% CI 0.76-0.92)
After assessing the performance of the StenoSCORE in our cohort, we then sought to determine if a different combination of variables within our cohort would better predict S. maltophilia BSI. Based on the results of univariable analysis, acute leukemia (acute myeloid or acute lymphoblastic leukemia), neutropenia ≥ 7 days, mucositis, ICU admission within 12 h of index culture, prior meropenem exposure ≥ 3 days, and prior cefepime exposure ≥ 3 days were selected for inclusion in the binary logistic regression model (Table S2). Neutropenia ≥ 7 days [OR 4.10 (95% CI 1.67–10.08)], mucositis [OR 2.51 (95% CI 1.08–5.79)], meropenem exposure [OR 4.63 (2.09–10.28)], and cefepime exposure [OR 2.55 (95% CI 1.14–5.69)] remained significant predictors of S. maltophilia BSI in the regression model. The model demonstrated good calibration with a Hosmer and Lemeshow chi-square 13.4, p-value = 0.06. Multicollinearity was not detected within the model as evidenced by variance inflation factors < 1.3 in regression analysis. Risk score points for the StenoSCORE2 are summarized in Table 1. Possible StenoSCORE2 values ranged from 0 to 8. The median StenoSCORE2 was 5 in the S. maltophilia BSI group compared with 1 in the non-S. maltophilia BSI group (p- value < 0.0001). ROC curve analysis of the StenoSCORE2 performance in our cohort produced an AUC 0.84 (95% CI 0.76–0.92) indicating excellent discrimination (Figure 1). Table 4 summarizes the sensitivity, specificity, and accuracy of the StenoSCORE2 at various point cutoffs. A StenoSCORE2 ≥ 4 had a sensitivity of 86%, specificity of 76%, and accurately identified 77% of S. maltophilia BSIs.
Discussion
In our cohort of patients with hematologic malignancy and/or HSCT, S. maltophilia BSI accounted for only 6.3% of Gram-negative BSI but was associated with significant in-hospital mortality. With in-hospital mortality rates of 58.3% seen in patients with S. maltophilia BSI compared with 18.5% in patients the non-S. maltophilia, our findings highlight the clinical importance of S. maltophilia BSI as a contributor to poor outcomes in this patient population. Recognition of risk factors for S. maltophilia BSI and prompt initiation of antimicrobial therapy active against S. maltophilia will be important to help close this wide mortality gap and improve outcomes in this vulnerable patient population.
Numerous studies have evaluated risk factors for S. maltophilia infection and BSI in patients with hematologic malignancy and/or HSCT [4, 9,10,11,12,13,14,15,16,17,18]. Our study was the first to systematically apply a collection of predetermined risk factors for S. maltophilia BSI known as the StenoSCORE and validate its utility in a second cohort of patients with hematologic malignancy and/or HSCT and Gram-negative BSI. To date, studies evaluating risk factors for S. maltophilia BSI have largely been single center or localized to a single health system [4, 9,10,11,12,13,14,15,16,17,18]. Regional differences in patient populations, oncologic treatment practices, and changes in treatment practices over time have led to heterogeneity among reported risk factors and outcomes for S. maltophilia BSI in patients with hematologic malignancy and/or HSCT. Consistent with the previous StenoSCORE publication, we identified characteristics which differentiate S. maltophilia BSI from non-S. maltophilia BSI in all patients with hematologic malignancy undergoing active chemotherapy and/or recent HSCT [4]. In contrast to another published risk scoring tool which aims to differentiate between S. maltophilia BSI and P. aeruginosa BSI in patients with hematologic malignancy, both the StenoSCORE and StenoSCORE2 can differentiate S. maltophilia BSI from BSI with Enterobacterales or other non-fermenters [12]. This is notable as the StenoSCORE and StenoSCORE2 may have greater generalizability given the greater prevalence of BSI caused by Enterobacterales as opposed to P. aeruginosa in this patient population as determined by the present study (Table S1) and others [19]. In our study, patients with S. maltophilia BSI had a median StenoSCORE of 42 which corroborated the previous report that StenoSCOREs ≥ 41 were able to discriminate S. maltophilia BSI from non-S. maltophilia BSI [4]. Additional multicenter students are warranted to confirm this finding.
Commonly cited risk factors for S. maltophilia BSI include carbapenem exposure, prolonged hospital stay, admission to the ICU, severe neutropenia, indwelling CVC, mucositis, and previous S. maltophilia colonization [4, 10,11,12, 14, 16,17,18]. Within our cohort, ANC at the time of index blood culture did not differ between patients with S. maltophilia BSI and non-S. maltophilia BSI. Additionally, the high proportion of patients within our cohort with an indwelling CVC at the time of index culture diminished the utility of evaluating CVC as a risk factor for S. maltophilia BSI. Thus, the resultant StenoSCORE2 derived from our cohort maintained acute leukemia, mucositis, and carbapenem exposure as important risk factors for S. maltophilia BSI but removed CVC and ANC as contributing risk variables (Table 1). Consistent with previous analyses, prolonged neutropenia and cefepime exposure were independent predictors of S. maltophilia BSI in multivariable analysis [10, 12, 17]. Additional variables found to be significantly associated with S. maltophilia BSI in univariable analysis yet not included in the multivariable analysis were increased time from hospital admission to culture, previous colonization or infection with S. maltophilia, piperacillin-tazobactam exposure, and use of antimicrobial prophylaxis. These variables were not selected for inclusion in the multivariable analysis either due to few patients with S. maltophilia BSI having each characteristic thus limiting analysis or expected collinearity with other variables included within the model. While the StenoSCORE exhibited acceptable performance within our cohort, optimization of the score to include variables determined to be clinically important risk factors within our patient population significantly increased the performance of the StenoSCORE2. Multicenter validation of the StenoSCORE and StenoSCORE2 is warranted to assess external validity of our findings.
While awaiting final culture results, the use of clinical scoring tools may help identify patients at increased risk for a particular infection with the intent of decreasing time to appropriate antimicrobial therapy [20]. This is of increasing importance in the absence of rapid molecular diagnostic testing capabilities where organism identification can take over 24 hours to result and likely contributes to delayed appropriate antimicrobial therapy [21]. Risk scoring tools for identification of S. maltophilia infection may be particularly useful in decreasing time to active antimicrobial therapy as S. maltophilia is intrinsically resistant to anti-pseudomonal β-lactams and aminoglycosides which are used as empiric therapy in immunocompromised patients with suspected infection. While risk scores have been developed to differentiate S. maltophilia BSI from non-S. maltophilia Gram-negative or P. aeruginosa BSI, literature describing implementation of these scoring systems into clinical practice and their impact on patient outcomes is lacking [4, 12]. Our results demonstrated a significantly longer time to appropriate antimicrobial therapy in patients with S. maltophilia BSI compared with patients with non-S. maltophilia BSI (0.57 h vs. 45.16 h; p < 0001) as well as delayed appropriate antimicrobial therapy as an independent predictor of in-hospital mortality. Future studies assessing the feasibility of implementation of the StenoSCORE and/or StenoSCORE2 into clinical practice and their impact on time to appropriate antimicrobial therapy and mortality are warranted.
This study has limitations to consider. Firstly, although there were relatively few cases with S. maltophilia BSI within our cohort which may limit statistical power to assess risk factors for S. maltophilia BSI, the present study represents one of the largest studies systematically assessing S. maltophilia risk scoring tools. Similarly, we found overall similar performance of the StenoSCORE in an independent cohort providing external validation to this pragmatic risk tool. The StenoSCORE2 has yet to be externally validated outside of the current cohort. Lastly, the increased mortality observed in the S. maltophilia BSI group was likely impacted by multiple factors. In addition to delayed initiation of active antimicrobials in the S. maltophilia BSI group, unmeasured confounders such as malignancy disease status and BSI source were not assessed. Recently, the efficacy of antimicrobials traditionally used as first-line therapy for S. maltophilia infections under the previous clinical breakpoints has also been under scrutiny which may also contribute to worse outcomes in this patient population [22,23,24]. Future studies are needed to evaluate optimal empiric and susceptibility-directed therapy for S. maltophilia BSIs.
In conclusion, the StenoSCORE and StenoSCORE2 performed well in predicting S. maltophilia BSI in a cohort of patients with hematologic malignancy and/or HSCT and Gram-negative BSI. Risk factor derivation from a multicenter study would improve the accuracy and generalizability of these findings. To improve patient outcomes in S. maltophilia BSI, clinical studies evaluating the StenoSCORE and/or StenoSCORE2 implementation on time to effective therapy and clinical outcomes are warranted.
Data availability
Data supporting the results of this study are available from the corresponding author upon reasonable request.
References
Boktour M, Hanna H, Ansari S, Bahna B, Hachem R et al (2006) Central venous catheter and Stenotrophomonas maltophilia bacteremia in cancer patients. Cancer 106:1967–1973. https://doi.org/10.1002/cncr.21846
Cho SY, Lee DG, Choi SM, Park C, Chun HS et al (2015) Stenotrophomonas maltophilia bloodstream infection in patients with hematologic malignancies: a retrospective study and in vitro activities of antimicrobial combinations. BMC Infect Dis 15:1–8. https://doi.org/10.1186/s12879-015-0801-7
Kim EJ, Kim YC, Ahn JY et al (2019) Risk factors for mortality in patients with Stenotrophomonas maltophilia bacteremia and clinical impact of quinolone-resistant strains. BMC Infect Dis 19:754. https://doi.org/10.1186/s12879-019-4394-4
Karaba SM, Goodman KE, Amoah J, Cosgrove SE, Tamma PD (2021) StenoSCORE: Predicting Stenotrophomonas maltophilia bloodstream infections in the hematologic malignancy population. Antimicrob Agents Chemother 65:e00793–e00721. https://doi.org/10.1128/AAC.00793-21
Banerjee R, Patel R (2023) Molecular diagnostics for genotypic detection of antibiotic resistance: current landscape and future directions. JAC Antimicrob Resist. https://doi.org/10.1093/jacamr/dlad018
CLSI (2022) Performance Standards for Antimicrobial Susceptibility Testing, 32nd edition. CLSI standard M100. Clinical and Laboratory Standards Institute. https://clsi.org/standards/products/microbiology/documents/m100/. Accessed 27 April 2023
Chotiprasitsakul D, Han JH, Cosgrove SE, Harris AD, Lautenbach E, Conley AT, Tolomeo P, Wise J, Tamma PD, Antibacterial Resistance Leadership Group (2018) Comparing the outcomes of adults with Enterobacteriaceae Bacteremia receiving short-course Versus prolonged-course antibiotic therapy in a Multicenter, Propensity score-matched cohort. Clin Infect Dis 66:172–177. https://doi.org/10.1093/cid/cix767
Gupta N, Limbago BM, Patel JB, Kallen AJ (2011) Carbapenem-resistant Enterobacteriaceae: epidemiology and prevention. Clin Infect Dis 53:60–67. https://doi.org/10.1093/cid/cir202
Saburi M, Oshima K, Takano K, Inoue Y, Harada K, Uchida N, Fukuda T, Doki N, Ikegame K, Matsuo Y, Katayama Y, Ozawa Y, Matsuoka KI, Kawakita T, Mori Y, Ara T, Nakamae H, Kimura T, Kanda Y, Atsuta Y, Ogata M, Transplant Complications Working Group of the Japanese Society for Transplantation and Cellular Therapy (2023) Risk factors and outcome of Stenotrophomonas maltophilia infection after allogeneic hematopoietic stem cell transplantation: JSTCT, Transplant complications Working Group. Ann Hematol 102:2507–2516. https://doi.org/10.1007/s00277-023-05320-4
Hotta G, Matsumura Y, Kato K, Nakano S, Yunoki T, Yamamoto M, Nagao M, Ito Y, Takakura S, Ichiyama S (2014) Risk factors and outcomes of Stenotrophomonas maltophilia bacteraemia: a comparison with bacteraemia caused by Pseudomonas aeruginosa and Acinetobacter species. PLoS ONE 9:e112208. https://doi.org/10.1371/journal.pone.0112208
Aitken SL, Sahasrabhojane PV, Kontoyiannis DP, Savidge TC, Arias CA, Ajami NJ, Shelburne SA, Galloway-Peña JR (2021) Alterations of the oral Microbiome and cumulative Carbapenem exposure are Associated with Stenotrophomonas maltophilia infection in patients with Acute myeloid leukemia receiving chemotherapy. Clin Infect Dis 72:1507–1513. https://doi.org/10.1093/cid/ciaa778
Sakoh T, Kimura M, Takagi S, Ogura S, Morishima M, Yamamuro R, Yamaguchi K, Yuasa M, Kaji D, Kageyama K, Taya Y, Nishida A, Ishiwata K, Yamamoto H, Yamamoto G, Asano-Mori Y, Wake A, Uchida N, Taniguchi S, Araoka H (2023) Predictive scoring system for distinguishing Stenotrophomonas maltophilia bacteremia from Pseudomonas aeruginosa bacteremia in patients with hematological malignancies. Ann Hematol 102:1239–1246. https://doi.org/10.1007/s00277-023-05185-7
Kim SH, Cho SY, Kang CI, Seok H, Huh K, Ha YE, Chung DR, Lee NY, Peck KR, Song JH (2018) Clinical predictors of Stenotrophomonas maltophilia bacteremia in adult patients with hematologic malignancy. Ann Hematol 97:343–350. https://doi.org/10.1007/s00277-017-3178-4
Garazi M, Singer C, Tai J, Ginocchio CC (2012) Bloodstream infections caused by Stenotrophomonas maltophilia: a seven-year review. J Hosp Infect 81:114–118. https://doi.org/10.1016/j.jhin.2012.02.008
Yeshurun M, Gafter-Gvili A, Thaler M, Keller N, Nagler A, Shimoni A (2010) Clinical characteristics of Stenotrophomonas maltophilia infection in hematopoietic stem cell transplantation recipients: a single center experience. Infection 38:211–215. https://doi.org/10.1007/s15010-010-0023-2
Lai CH, Wong WW, Chin C, Huang CK, Lin HH, Chen WF, Yu KW, Liu CY (2006) Central venous catheter-related Stenotrophomonas maltophilia bacteraemia and associated relapsing bacteraemia in haematology and oncology patients. Clin Microbiol Infect 12:986–991. https://doi.org/10.1111/j.1469-0691.2006.01511.x
Labarca JA, Leber AL, Kern VL, Territo MC, Brankovic LE, Bruckner DA, Pegues DA (2000) Outbreak of Stenotrophomonas maltophilia bacteremia in allogenic bone marrow transplant patients: role of severe neutropenia and mucositis. Clin Infect Dis 30:195–197. https://doi.org/10.1086/313591
Apisarnthanarak A, Mayfield JL, Garison T, McLendon PM, DiPersio JF, Fraser VJ, Polish LB (2003) Risk factors for Stenotrophomonas maltophilia bacteremia in oncology patients: a case-control study. Infect Control Hosp Epidemiol 24:269–274. https://doi.org/10.1086/502197
Zimmer AJ, Stohs E, Meza J, Arnold C, Baddley JW, Chandrasekar P, El Boghdadly Z, Gomez CA, Maziarz EK, Montoya JG, Pergam S, Rolston KV, Satlin MJ, Satyanarayana G, Shoham S, Strasfeld L, Taplitz R, Walsh TJ, Young JH, Zhang Y, Freifeld AG (2022) Bloodstream infections in hematologic malignancy patients with fever and Neutropenia: are empirical antibiotic therapies in the United States still effective? Open Forum Infect Dis 9:ofac240. https://doi.org/10.1093/ofid/ofac240
Haimerl BJ, Encinas R, Justo JA, Kohn J, Bookstaver PB, Winders HR, Al-Hasan MN (2023) Optimization of empirical antimicrobial therapy in Enterobacterales Bloodstream infection using the extended-spectrum beta-lactamase prediction score. Antibiot (Basel) 12:1003. https://doi.org/10.3390/antibiotics12061003
Dodémont M, De Mendonça R, Nonhoff C, Roisin S, Denis O (2014) Performance of the Verigene Gram-negative blood culture assay for rapid detection of bacteria and resistance determinants. J Clin Microbiol 52:3085–3087. https://doi.org/10.1128/JCM.01099-14
Lasko MJ, Tabor-Rennie JL, Nicolau DP, Kuti JL (2022) Trimethoprim/sulfamethoxazole pharmacodynamics against Stenotrophomonas maltophilia in the in vitro chemostat model. J Antimicrob Chemother 77:3187–3193. https://doi.org/10.1093/jac/dkac304
Fratoni AJ, Nicolau DP, Kuti JL (2021) Levofloxacin pharmacodynamics against Stenotrophomonas maltophilia in a neutropenic murine thigh infection model: implications for susceptibility breakpoint revision. J Antimicrob Chemother 77:164–168. https://doi.org/10.1093/jac/dkab344
Fratoni AJ, Nicolau DP, Kuti JL (2022) Minocycline pharmacodynamics against Stenotrophomonas maltophilia in the neutropenic murine infection model: implications for susceptibility breakpoints. J Antimicrob Chemother 77:1052–1060. https://doi.org/10.1093/jac/dkac018
Acknowledgements
The authors would like to thank Melissa Beasely, MS who assisted with data collection.
Funding
This study was funded by a Barnes Jewish Foundation grant awarded to CM.
Author information
Authors and Affiliations
Contributions
Study concept and design: ELG, CMG, and CM. Statistical analysis: ELG and CMG. Analysis and interpretation of data: ELG, CMG, and CM. First draft of the manuscript: ELG. Critical revision of the manuscript: ELG, CMG, and CM. Contributed intellectual material and approved final draft: ELG, CMG, and CM.
Corresponding author
Ethics declarations
Ethical approval
This study was approved by the Washington University in St. Louis Institutional Review Board (IRB ID: 202201035).
Competing interests
CMG has received research funding from Cepheid, Everest Medicines, Shionogi, and Entasis. ELG and CM have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gill, E.L., Gill, C.M. & McEvoy, C. Validation of a Stenotrophomonas maltophilia bloodstream infection prediction score in the hematologic malignancy population. Ann Hematol 103, 1745–1752 (2024). https://doi.org/10.1007/s00277-024-05686-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-024-05686-z